Ziwei Gao
Shaanxi Normal University, China
Title: Multi-functional N, O donors for Cu catalyzed Csp2-Csp cross-coupling reaction
Biography
Biography: Ziwei Gao
Abstract
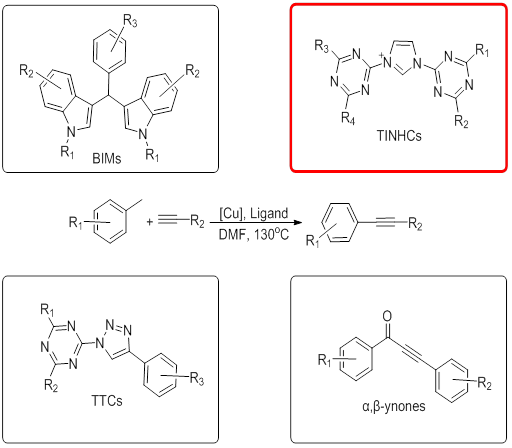
The Sonogashira reaction is one of the most important and widely used Csp2–Csp bond formation reactions in organic synthesis, frequently employed in the synthesis of natural products, biologically active molecules, heterocycles and molecular nanostructures. Various attempts have been made to achieve the Sonogashira reaction in the absence of the expensive noble metal palladium, and copper has proved an excellent alternative. With intensive study of the copper-based catalyst system, it is found that the coupling efficiency is greatly enhanced by 2e electron donor ligands. Since Miura and co-workers introduced the CuI/PPh3-catalyzed cross-couplings of aryl iodides and aryl acetylenes, hard donors as green and efficient promoters have dominated the copper-catalyzed coupling reactions instead of the classic phosphine ligands. Recently, we developed four kinds of multi-functional ligands which significantly enhanced the activity of low-mol% (1-5%) copper precatalysts. N, O donors ligands of bisindoles (BIMs), Triazine-Triazole conjugates (TTCs), Triazine-imidazole N-heterocyclic carbenes (TINHCs) and ynones have ensured the robust and straight forward cu catalyzed cross-coupling of Csp2-Csp bond, making this an indispensable methodology for the synthesis of complex molecular architectures of C≡C. These ligands inherit both the stability and activity of triazines and aromatic system, which were easily synthesized by multi-step nucleophilic substitution reaction.