
Zhongyi Ma
Chinese Academy of Sciences, China
Title: Comparative study of CO adsorption on zirconia polymorphs by diffuse reflectance FT-IR and transmission FT-IR spectroscopy
Biography
Biography: Zhongyi Ma
Abstract
Interests in the surface properties of zirconia aroused a steady interest over many years because this solid exhibited catalytic behavior in dehydration, hydrogenation, and hydrogen exchange reactions. A great deal of work has been devoted to the study of zirconia, as both a catalyst and a support for metal catalysts. In this study the interactions of CO with amorphous zirconia (am-ZrO2), monoclinic zirconia (ZrO2), and tetragonal zirconia (t-ZrO2) were investigated by diffuse reflectance FT-IR spectroscopy and transmission FT-IR to determine the influence of zirconia polymorphs on CO adsorption and conversion. Moreover, the effects of dehydroxylation degree of different zirconia polymorphs on CO adsorption at ambient temperature was also surveyed at different thermal vacuum treatments were observed by transmission FT-IR. The results showed that zirconia polymorphs exerted a great influence on CO adsorption, the formation and conversion of surface intermediates, and the active sites for CO adsorption varied with the dehydroxylation degree of zirconia polymorphs. CO bands were detected on am-ZrO2 and m-ZrO2 samples either by CO adsorption at high temperature or CO adsorbed on dehydroxylated samples. The former was achieved by formation of formate species at high temperature via CO reacting with surface hydroxyl. The latter achievement was accomplished by the thermal dehydroxylation to decrease the surface coordinative water and hydroxyl. While in the case of t-ZrO2 sample, CO band was only created by the formation of bicarbonate species at high temperature to produce the cationic sites. The active sits for CO adsorption were created by CO reacting with surface hydroxyls at elevated temperature in the case of DRIFT spectra, while the active sites were produced by the thermal dehydroxylation in transmission FT-IR spectra.
A) 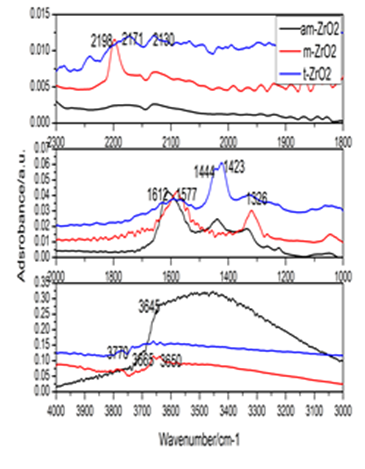
 B)
B)
Fig.1: FT-IR of CO adsorption on ZrO2 polymorphs. A: DRIFT spectra; B: Difference transmission infrared spectra

