
Yuichi Shimazaki
Ibaraki University, Japan
Title: Mechanistic study of alcohol oxidation by oxidized CuII salen-type complexes: Relationship between reactivity and the electronic structure of the complexes
Biography
Biography: Yuichi Shimazaki
Abstract
The redox chemistry of metal complexes has been widely developed in recent years, affording deep insights into the reaction mechanisms for many useful homogeneous catalytic reactions and enzymatic reactions at the active site of metalloenzymes. While studies on oxidative reaction intermediates are in progress, the oxidation state of the metal ions in the active species has not been fully understood until now. Detailed descriptions of the oxidation state of the intermediate are sometimes complicated, because the oxidation locus on oxidized metal complexes is often different from the “formal” oxidation site. Although “formal” and “experimental” oxidation numbers are identical in many cases, they are frequently used as synonyms, since the term of the physical or experimental oxidation state has not been accepted in some areas of chemistry. This practice leads to considerable confusion and presents an obstacle to the progress of the experimental determination of the intermediate. Tetradentate Schiff-bases, such as salens, have received considerable attention as a ligand system due to their relative ease of synthesis, ability to form stable complexes with many metals in a variety of oxidation states, and versatility as catalysts for important organic transformations. Concurrent studies have investigated the electronic structure of oxidized metal salen systems, determining the factors that control the locus of oxidation in these complexes. Depending on the relative energies of the redox-active orbitals, metal complexes with pro-radical ligands can exist in a limiting description as a metal-ligand radical, [Mn+(L·)]n+, or a high valent metal complex, [M(n+1)+(L-)]n+. This presentation would discuss with recent advances in the chemistry related with the oxidation behavior of some metal–salen type complexes and their properties, especially focusing on comparison of their properties with those of the high valent metal–phenolate complexes which are iso-electronic with the phenoxyl radical complexes.
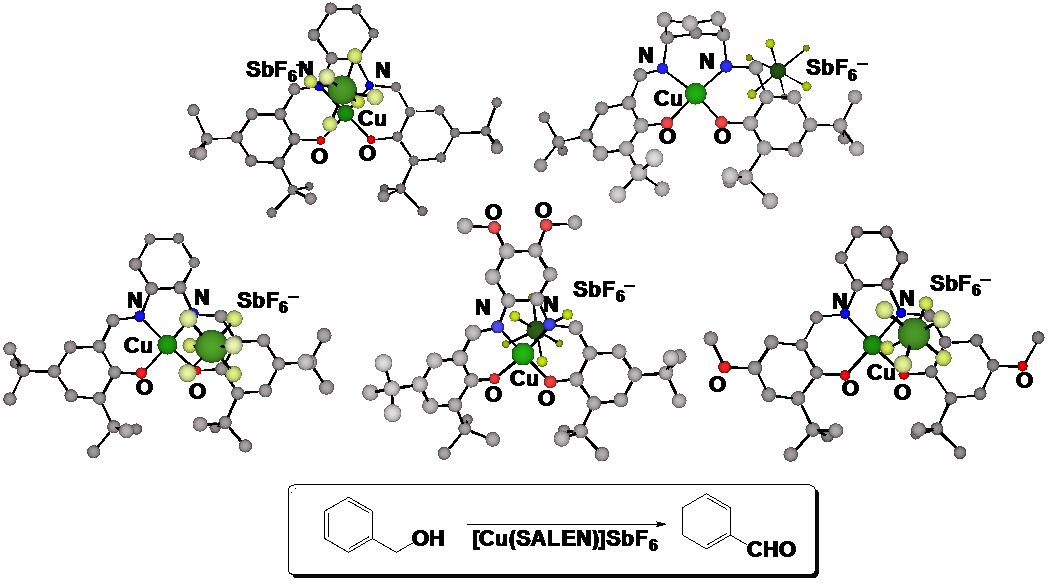
Figure 1: Crystal structures of one-electron oxidized Cu(II)-salen type complexes and the oxidation of benzyl alcohol by their oxidized complexes.

