
Nawarat Worauaychai
Ramkhamhaeng University, Thailand
Title: Corrosion behavior of TiC-Ni-5Mo2C cermet
Biography
Biography: Nawarat Worauaychai
Abstract
Corrosion in TiC-Ni-5Mo2C cermet was studied in 0.3M H2SO4, 1M NaCl and 1M NaOH by means of anodic polarization method. Corrosion morphologies were then examined by SEM-EDS. The results showed that there are two ranges of passive region in acidic condition; whereas only one passive region was observed in alkaline solution. In case of chloride containing solution, passive region was hardly observed. The SEM micrographs revealed that nickel binder was a corrosion onset site in acidic and salt solution. In contrast to alkaline condition, the deterioration commenced at TiC. The driving force for corrosion susceptibility was the micro-galvanic cell formation between TiC and Nickel.
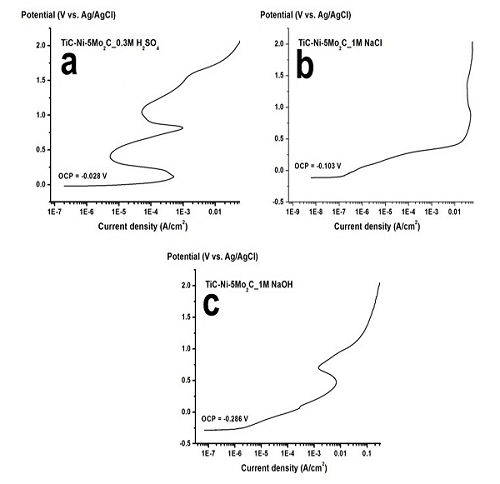
Figure 1: Potentiodynamic polarization curves of TiC-Ni-5Mo2C cermet in (a) 0.3M H2SO4 (b) 1M NaCl (c) 1M NaOH solution

