
Sara Arafeh
Marquette University, USA
Title: Quantitative detection of the conformational transitions between open and closed forms of cytochrome P450 oxidoreductase (CYPOR) at the membrane surface in different functional states
Biography
Biography: Sara Arafeh
Abstract
Cytochrome P450 and monooxgenases require a supply of electrons to catalyze the synthesis of steroid hormones, fatty acids and prostaglandin hormone. Cytochrome P450 oxidoreductase (CYPOR), a membrane bound enzyme, provides these electrons in its open conformation. CYPOR has two cytosolic domains (FAD domain and FMN domain) and an N-terminal in the membrane (Figure 1). In its open conformation, electrons flow from NADPH, FAD, and finally to FMN where cytochrome P450 picks up these electrons. In the closed conformation, cytochrome P450 does not bind to the FMN domain to take the electrons (Figure 2). It was found that when the cytosolic domains are isolated, CYPOR cannot bind to cytochrome P450. This suggested that the membrane environment is important for CYPOR function. Studies on the open/closed conformations of the full-length CYPOR have never been done, so this project takes the initiative to better understand the function of CYPOR in its full length. Here, we determine the distance between specific sites in the FAD and FMN binding domains in CYPOR by Forster Resonance Energy Transfer (FRET) and Ultrafast TA spectroscopy with and without NADPH. The approach to determine these distances will rely on labeling these sites with red and infrared fluorophores. Mimic membrane attachment is done by inserting CYPOR in lipid nanodiscs. By determining the distances between the donor-acceptor sites in these domains, we can observe the open/closed conformations upon reducing CYPOR in the presence and absence of cytochrome P450. Such study is important to better understand CYPOR mechanism of action in various endosomal membranes including hepatic CYPOR which is vital in plasma cholesterol homeostasis. By investigating the conformational cycles of CYPOR, we can synthesize drugs that would be more efficient in affecting the steroid hormonal levels in human cells.
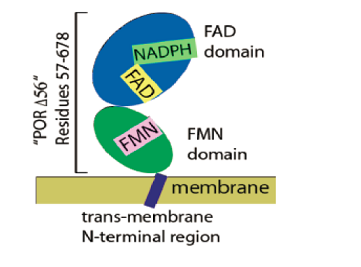
Figure 1: CYPOR has two parts : Cytosolic and membrane bound. There are two domains in the cytosolic part which are the FAD and FMN domains. CYPOR binds to membrane via its N-terminal region.

