Marco Giulio Rigamonti
Politecnico di Milano, Italy
Title: Stereoselective synthesis of hernandulcin, peroxylippidulcine A, lippidulcines A, B and C and taste evaluation
Biography
Biography: Marco Giulio Rigamonti
Abstract
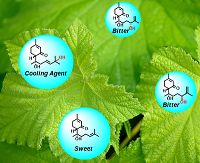
Sucrose abuse is strongly associated to many undesirable health effects. Artificial sweeteners are a popular alternative but many raise questions regarding their safety. The Mexican plant Lippia dulcis contains trace amounts of (+)-hernandulcin, a compound so sweet – 1000 times more that sucrose–that few leaves are enough to sweeten a cup of tea. Recent studies revealed that the plant also produces several derivatives of this molecule: The peroxylippidulcines and the lippidulcines A, B and C. However, these sesquiterpenes have been isolated in such a small amount that it has not been possible to assess their taste. A multigram scale-up and optimization for the synthetic route of (+)-hernandulcin, allowed us to accomplish the first stereoselective synthesis of lippidulcines A, B and C. With modified version of the Kornblum–DeLaMare rearrangement, and a highly regioselective and stereoselective ketone reduction with the MeCBS reagent we synthesized the compounds and confirmed the previously assigned absolute configuration. The taste evaluations indicate that lippidulcine A is a cooling agent with a mint after taste, while none of these sesquiterpenes are sweet. Indeed, the insertion of a hydroxy group on the side chain of hernadulcin annuls its intense sweetness.