Anil K Tripathi
Indian Institute of Integrative Medicine (CSIR), India
Title: Synthesis of 7-(2-substituted-phenylthiazolidinyl)-benzopyran-2-one derivatives
Biography
Biography: Anil K Tripathi
Abstract
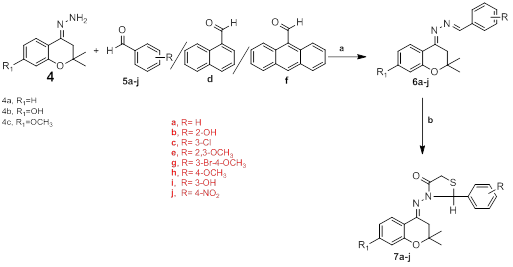
The molecular exploitation of promising lead compounds is still a major line of approach to develop new drugs. It involves an effort to combine the separate pharmacophoric groups of similar activity into one compound, thus making structural changes in the biological activity. Thiazolidinone ring present in a large number of biologically active molecules of different pharmacological classes exhibited different activities. The historical importance of thiazolidine derivative was emphasized during the period 1941–1945, i.e. the development of penicillin which shows the presence of thiazoline ring. The discovery of benzopyran like derivatives as therapeutic agents in early 1820’s was the beginning of the chroman related drug development. The chroman derivatives have a relatively broad spectrum with high activity proï¬le against various bacteria and fungi. Due to the diversiï¬ed nature of chroman and thiazolidinone, which render them to be useful substances in drug researches. In continuation of our search for novel biologically active benzopyran derivatives, in this paper we report the synthesis and antibacterial/anti-cancer/anti-inflammatory activity of a novel series of Schiff bases of 2, 2-dimethyl-4-hydrazone chromanone and its derivatives 4-substituted phenylthiazolidinyl chroman derivatives. The synthetic strategy is delineated in Scheme 1. Numerous keto compounds reacted directly with hydrazine hydrate in ethanol to obtain substituted hydrozones which was further treated with various formyl derivatives in absolute alcohol using catalytic amounts of acetic anhydride to afford substituted shiff’s bases. To conclude this particular reaction sequence finally, treated the shiff’s bases with thioglycolic acid in dioxan in presence of zinc chloride we obtained substituted phenylthiazolidinyl chroman derivatives (7a-j). All the synthesized compounds were purified by column chromatography before characterization by spectroscopic methods.