
Wei Zhang
Chinese Academy of Sciences, China
Title: The synthesization of SAPO-11 and its catalytic performance for the alkylation of naphthalene
Biography
Biography: Wei Zhang
Abstract
The SAPO-11 zeolite is a member of the silico-aluminophosphate (SAPO-n) family which was first synthesized by Union Carbide Corporation. It has novel pore structures and exhibits milder acidity due to the presence of phosphorus. The pore size of SAPO-11 was more than 0.60nm, which can sieve effectively the products of the methylation of naphthalene. In this study, SAPO-11 zeolites were successfully synthesized by using three different templates (diethylamine (DEA), di-n-propylamine (DPA) and di-iso-propylamine (DIPA)) under hydrothermal conditions. The structures and acidities of SAPO-11 were characterized by means of XRD, BET and NH3-TPD. XRD results indicated that the directing effect of different templates for AEL structure decreased in the order of DPA >DEA>DIPA. N2 adsorption-desorption results showed that SAPO-11(DPA, 1.2) possesses higher surface area and larger pore volume than other samples, which corresponds to the higher crystallinity. This is in agreement with the results of the XRD.
The alkylation of naphthalene (NAPH) with methanol (ME) on the SAPO-11 zeolite was investigated. 1, 3, 5-trimethylbenzene (TMB) was used as solvent. The effects of the reactant mol ratio, the reaction temperature and the weight hourly space velocity (WHSV) on the conversion of NAPH and the selectivity to 2, 6-dimethylnaphthalene (2, 6-DMN) was studied. The results showed that the optimum reaction conditions are as follows: 0.1 MPa, 425℃, n(NAPH)∶n(ME)∶n(TMB)=1∶5∶3.5, WHSV 0.06 h−1.
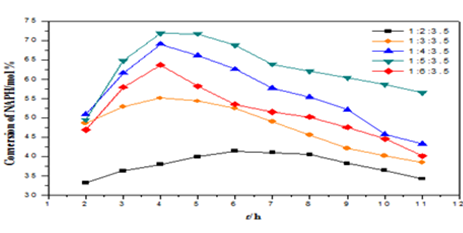
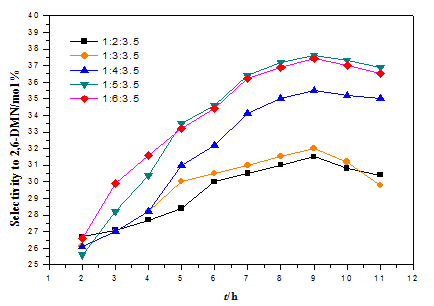
Fig.1: The effect of reactant mol ratio on the conversion of NAPH and the selectivity to 2, 6-DMN

