Majid M Heravi
Alzahra University, Iran
Title: Diketene-based multicomponent reaction strategy toward N-heterocycles
Biography
Biography: Majid M Heravi
Abstract
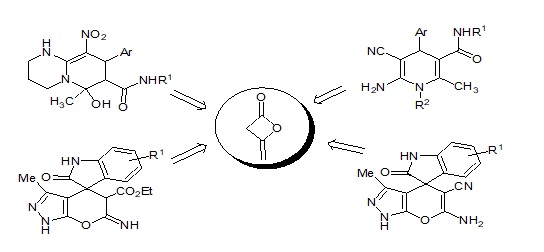
Diketene (4-methylene-oxetan-2-one or DK) consists of a four-membered lactone ring adjacent to a methylene function and it can be considered the anhydride of acetoacetic acid. DK is a reactive, readily available, and versatile molecule. Diketene also reacts with alcohols and amines to the corresponding acetoacetic acid derivative. DK appears to be an ideal molecule to be used in organic transformations. Diketene possesses electrophilic and nucleophilic sites which are capable of undergoing numerous reactions. In 1986 the chemistry of DK has been extensively and comprehensively reviewed by R.J Clemens. In the last decade, various interesting multicomponent reactions based on DK successfully achieved leading to the construction of a wide variety of heterocyclic systems. Very recently, we also published on the applications of DK as a privileged synthon in the synthesis of heterocyclic compounds as a chapter in Advances in Heterocyclic Chemistry. Continuing our efforts in the development of multicomponent reaction for the synthesis of potential biological activity nitrogen-containing heterocycles, in the present work, we describe the efficient synthesis of poly-substituted nitrogen-containing heterocycles, such as 1,4-dihydropyridines, pyrido[1,2-a] pyrimidines and spiro[indoline-3,4'-pyrano-pyrazole] derivatives via MCRs involving diketene.