
Ali Mohd Lone
Govt. Degree College for Women Baramulla, India
Title: Divergent approaches to the synthesis of fused furo[3,2-b]furanone scaffold embodied in bioactive natural products
Biography
Biography: Ali Mohd Lone
Abstract
Furo-furanones are the bicyclic compounds in which furan ring is attached to a furanone ring through face-b. Functionally embellished furo[3,2-b]furanone moiety has been widely encountered as a distinctive sub-structure among a diverse range of natural products of mixed biosynthetic origin (Figure). Representative examples of natural products incorporating the furo[3,2-b]furanone segment as part of their complex architecture are neovibsanins A and B), lactonamycin, schisandra nortriterpenoids (e.g., micrandilactone A), plumericin, pallavicinin and neopallavicinin and plakortones (e.g., plakortone E). All these natural products exhibit wide-ranging biological activities. Thus, the furo[3,2-b]furanone core appears to be a promising and potent pharmacophoric group that imparts considerable therapeutic value to the natural products that embody it. This attribute along with the complex, challenging and diverse natural product architecture into which furo[3,2-b]furanone core is embedded, has generated considerable interest in assembling this moiety. In the present study, a simple and straightforward methodology of general utility to construct sterically encumbered furo[3,2-b]furanone scaffolds present in a diverse range of bioactive natural products is delineated. The methodology emanates from readily available Morita–Baylis–Hillman (MBH) adducts and employs sequential ring closing metathesis and oxy-Michael addition cascade as the key steps.
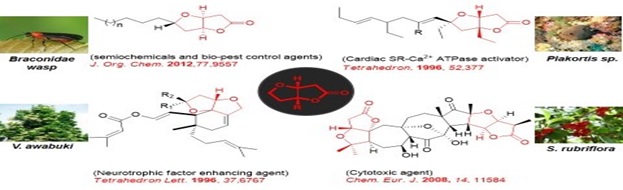
Figure. SAR of 2-alkyl/aryl-aminomethylene cycloalkane-1,3-diones against M. Tuberculosis.

