Edward Lee Ruff
York University, Canada
Title: Preparation of novel nucleoside analogues from cyclobutane precursors as potential antiviral agents
Biography
Biography: Edward Lee Ruff
Abstract
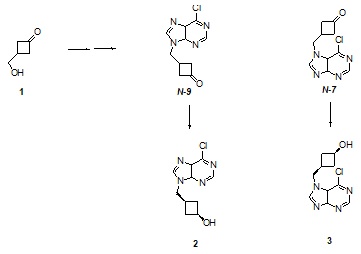
Cyclobutanes represent strained compounds which exhibit chemical reactivity not encountered with unstrained ring systems. These properties have been exploited in their capacity as synthetic intermediates. Cyclobutane nucleosides as oxetanocin analogs have been shown to exhibit antiviral and other biological activities.1 Our interests in cyclobutanone chemistry has prompted investigations into the preparation of novel cyclobutane nucleoside analogs. We report in this paper the synthesis of novel cyclobutanols 2 and 3 from its precursor 1. The coupling of 6-chloropurine with 1 gives two regioisomers consisting of the N-9 and N-7 ketones with the latter formed as the major product.. These were selectively reduced using a hindered hydride donor to