Teresa Pinho e Melo
University of Coimbra, Portugal
Title: The chemistry of nitrosoalkenes and azoalkenes in the synthesis of heterocyclic compounds
Biography
Biography: Teresa Pinho e Melo
Abstract
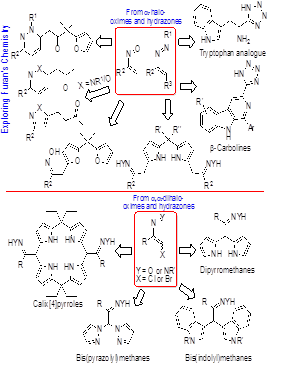
The chemistry of conjugated nitrosoalkenes and azoalkenes, used as electron-deficient heterodienes in hetero-Diels–Alder reactions or as Michael-type acceptors in conjugated 1, 4-addition reactions, has been successfully explored for the synthesis of a plethora of heterocyclic systems (Figure 1). Hetero-Diels–Alder reactions of 3-tetrazolyl- nitrosoalkenes and azoalkenes proved to be an efficient approach to 5-(substituted)-1H-tetrazoles, including tryptophan analogues and β-carbolines bearing the 1H-tetrazol-5-yl substituent. The alkylation of five-membered heterocyclic compounds via reaction with conjugated nitrosoalkenes and azoalkenes was also applied to the functionalization of dipyrromethane and bis(furan-2-yl)methanes. Combining hetero-Diels–Alder reactions of nitroso- and azoalkenes with furans with their ring-opening reactions, the synthesis of novel heterocycles was achieved, namely 6-(2-oxobutyl)-1,6-dihydropyridazines, 6-(2-oxopropyl)-1,6-dihydropyridazines, 5-(3-oxobutyl)-isoxazoles and 5-(3-oxobutyl)-pyrazoles. Base-mediated dehydrohalogenation of α,α-dihalo-oximes and α,α-dihalo-hydrazones in the presence of pyrrole, indole and pyrazoles allowed the development of new routes to dipyrromethanes, bis(indolyl)methanes and bis(pyrazolyl)methanes, respectively. Calix [4]pyrroles and bilanes were also obtained from the reaction of azoalkenes with dipyrromethanes. The study included the biological evaluation of some of the new heterocyles. Further details of this chemistry will be presented and discussed.
Image
Recent Publications
1. a) Lopes, S. M. M.; Palacios, F.; Lemos, A.; Pinho e Melo, T. M. V. D. Tetrahedron 2011, 67, 8902. b) Lopes, S. M. M.; Brigas, A. F.; Palacios, F.; Lemos, A.; Pinho e Melo, T. M. V. D. Eur. J. Org. Chem. 2012, 2152.
2. a) Lopes, S. M. M.; Lemos, A.; Pinho e Melo, T. M. V. D. Eur. J. Org. Chem. 2014, 7039. b) Nunes, S. C. C.; Lopes, S. M. M.; Gomes, C. S. B.; Lemos, A.; Pais, A. A. C. C.; Pinho e Melo, T. M. V. D. J. Org. Chem. 2014, 79, 10456. c) Jorda, R.; Lopes, S. M. M.; ŘezníÄková, E.; Kryštof, V.; Pinho e Melo, T. M. V. D. ChemMedChem 2017, 12, 701.
3. a) Lopes, S. M. M.; Henriques, M. S. C.; Paixão, J. A.; Pinho e Melo, T. M. V. D. Eur. J. Org. Chem. 2015, 6146. b) Alves, A. J. S.; Lopes, S. M. M.; Henriques, M. S. C.; Paixão, J. A.; Pinho e Melo, T. M. V. D. Eur. J. Org. Chem. 2017, 4011.
4. Pereira, N. A. M.; Lopes, S. M. M.; Lemos, A.; Pinho e Melo, T. M. V. D. Synlett 2014, 25, 423.
5. a) Grosso, C.; Cardoso, A. L.; Lemos, A.; Varela, J.; Rodrigues, M. J.; Custódio, L.; Barreira, L.; Pinho e Melo, T. M. V. D., Eur. J. Med. Chem. 2015, 93, 9. b) Grosso, C.; Cardoso, A. L.; Rodrigues, M. J.; Marques, C.; Barreira, L.; Lemos, A.; Pinho e Melo, T. M. V. D. Bioorganic & Medicinal Chemistry 2017, 25, 1122.
6. Grosso, C.; Lemos, A.; Pinho e Melo, T. M. V. D. Synlett 2014, 25, 2868.