Day :
- Sessions/ Tracks:
Advanced Synthesis and Catalysis | Bioorganic and Biochemistry | Organometallic Chemistry | Advance Trends in Organic Chemistry | Natural Products and Heterocyclic Chemistry | Modern Organic Chemistry and Applications | Medicinal Chemistry, Drug Synthesis | Inorganic Materials and Nanoparticles | Green Chemistry and Sustainable Technology | Polymer Chemistry

Chair
Yingju Xu
Merck & Co.

Co-Chair
Hyun-Joon Ha
Hankuk University of Foreign Studies, Korea
Session Introduction
Yuichi Shimazaki
Ibaraki University, Japan
Title: Mechanistic study of alcohol oxidation by oxidized CuII salen-type complexes: Relationship between reactivity and the electronic structure of the complexes
Time : 11:15-11:40

Biography:
Yuichi Shimazaki received his Doctor’s degree in Science from Nagoya University in 2000 under the supervision of Professor Osamu Yamauchi. He joined Professor Yoshinori Naruta’s group at Kyushu University as Assistant Professor and worked on the redox behavior of various metal porphyrin complexes as models of the active site of metalloenzymes. In 2008, he was promoted to Associate Professor at the College of Science, Ibaraki University. His research interests include the oxidation chemistry of the complexes of various metal ions, model studies of metalloenzymes, bioorganometallic chemistry and weak interactions in metal-organic molecule systems.
Abstract:
The redox chemistry of metal complexes has been widely developed in recent years, affording deep insights into the reaction mechanisms for many useful homogeneous catalytic reactions and enzymatic reactions at the active site of metalloenzymes. While studies on oxidative reaction intermediates are in progress, the oxidation state of the metal ions in the active species has not been fully understood until now. Detailed descriptions of the oxidation state of the intermediate are sometimes complicated, because the oxidation locus on oxidized metal complexes is often different from the “formal” oxidation site. Although “formal” and “experimental” oxidation numbers are identical in many cases, they are frequently used as synonyms, since the term of the physical or experimental oxidation state has not been accepted in some areas of chemistry. This practice leads to considerable confusion and presents an obstacle to the progress of the experimental determination of the intermediate. Tetradentate Schiff-bases, such as salens, have received considerable attention as a ligand system due to their relative ease of synthesis, ability to form stable complexes with many metals in a variety of oxidation states, and versatility as catalysts for important organic transformations. Concurrent studies have investigated the electronic structure of oxidized metal salen systems, determining the factors that control the locus of oxidation in these complexes. Depending on the relative energies of the redox-active orbitals, metal complexes with pro-radical ligands can exist in a limiting description as a metal-ligand radical, [Mn+(L·)]n+, or a high valent metal complex, [M(n+1)+(L-)]n+. This presentation would discuss with recent advances in the chemistry related with the oxidation behavior of some metal–salen type complexes and their properties, especially focusing on comparison of their properties with those of the high valent metal–phenolate complexes which are iso-electronic with the phenoxyl radical complexes.
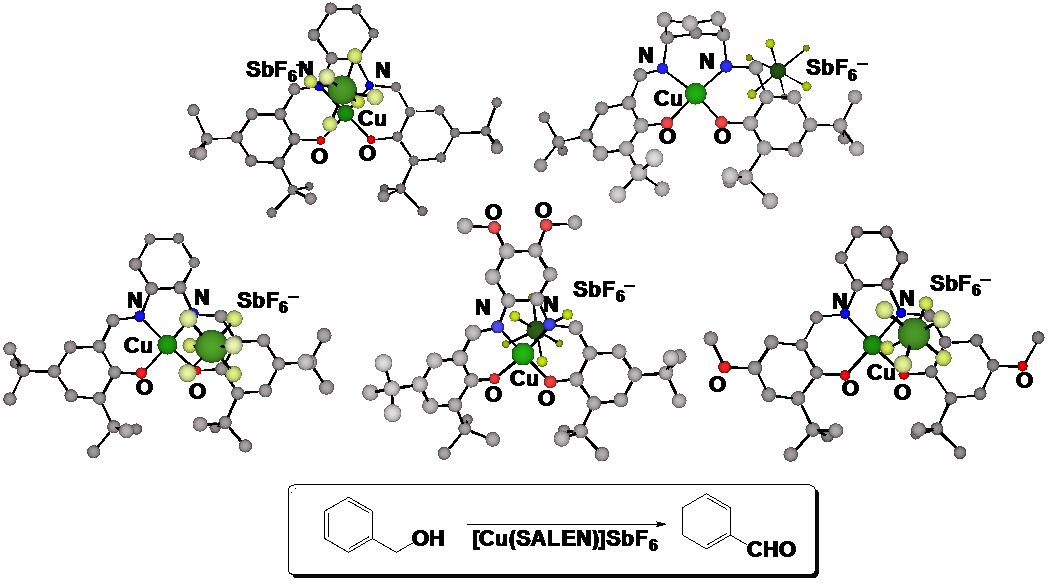
Figure 1: Crystal structures of one-electron oxidized Cu(II)-salen type complexes and the oxidation of benzyl alcohol by their oxidized complexes.
Ashoka Samuelson
Indian Institute of Science, India
Title: Electronic requirements for C-H activation in half-sandwich complexes
Time : 11:40-12:05

Biography:
Ashoka G Samuelson had his early education in Tiruchirapalli, India. After obtaining a Master’s degree in Chemistry from the Indian Institute of Technology, Madras at Chennai he finished his Doctoral studies at Cornell University, Ithaca. He has been working in the Department of Inorganic and Physical Chemistry at the Indian Institute of Science, Bangalore since then. His research interests are organometallic and coordination chemistry of transition metals. Their goal is to utilize the reactivity of organometallic compounds to devise new catalysts or anticancer active molecules. His research group often employs computational tools to supplement and complement their experimental findings.
Abstract:
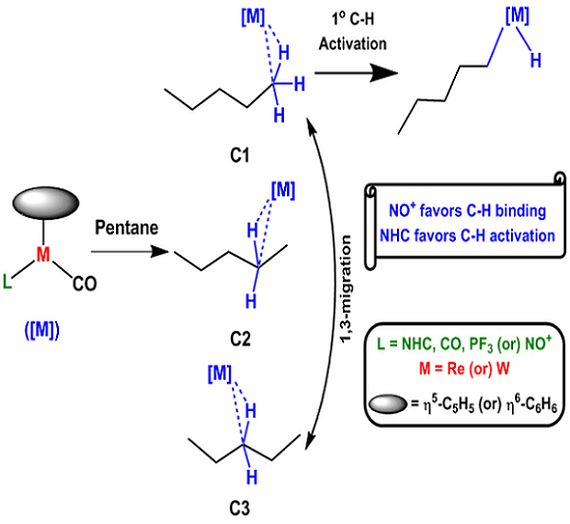
Activation of C-H bonds continues to attract significant attention. The interaction of C-H bonds plays an important role in several reactions besides being the key step for activating alkanes. We have carried out computational study of the interaction half-sandwich metal fragments (metal=Re/W/Ru, electron count=d6/d8), containing linear nitrosyl (NO+), carbon monoxide (CO), trifluorophosphine (PF3), N-heterocyclic carbene (NHC) ligands with alkanes using density functional theory employing the hybrid meta-GGA functional (M06). With d6 metal complexes, electron deficiency on the metal improves the formation of stable alkane complexes in the order NHC
Ling He
Xi’an Jiaotong University, China
Title: Hydrophobic, hydrophilic and amphiphilic silica/copolymer nanoparticles by surface-initiated atom transfer radical polymerization
Time : 12:05-12:30

Biography:
Ling He is a Professor and Director of Chemistry Department, Xian Jiaotong University. She got her PhD in Material Chemistry and MSc in Analytical Chemistry in China. She has worked as Visiting Scholar in University of Munich University (Germany) and University of Bologna (Italy). The main research fields are focused on copolymers and their self-assembly micro/nano hierarchical properties for coating materials, and the characterization of polychromy and analysis of natural polymeric compounds by nondestructive and in-situ detecting techniques. The main research activities includes hosting the National Key Basic Research and Development Program (973 program), Natural Science Foundation of China (NSFC) program; Key Project in the National Science and Technology Pillar Program of China. During the last five years, she has published about 80 articles in peer reviewed journals, published 5 books and made many presentations as well as invited plenary lectures in international meetings and conferences.
Abstract:
Silica/diblock copolymer nanoparticles (SDCNs) are synthesized by surface-initiated atom transfer radical polymerization (SI-ATRP) for hydrophobic, hydrophilic and amphiphilic materials, their properties as protein-resistance coatings are investigated. The hydrophobic SDCNs of SiO2-g-PMMA-b-P12FMA is synthesized by methyl methacrylate (MMA) and dodecafluoroheptyl methacrylate (12FMA) using silica surface initiator (SiO2-initiator with a density of 0.573 mmol·g-1). The three mass rations of hydrophobic SDCNs are proved as 25–30 nm core–shell particles in CHCl3 solution composed of P12FMA core and PMMA shell, but densely twined together as agglomerated particles. The increasing P12FMA content could obviously increase the surface roughness of the film (50-500 nm) and thereby contributes to the hydrophobic (112-118º) and oleophobic (45-78º) properties with lower water absorption and viscoelasticity, but a high thermo stability at 420-450℃. The amphiphilic SDCNs of SiO2-g-P(PEGMA)-b-P(12FMA) is prepared by poly (ethylene glycol) methyl ether methacrylate (PEGMA) and P12FMA. Their amphiphilic behavior, lower critical solution temperature (LCST), and surface properties as protein-resistance coatings are characterized. The individual spherical nanoparticles (150 nm -170 nm) as P(PEGMA)-b-P(12FMA) shell grafted on SiO2 core (130 nm) to gain obvious low LCST at 36-52℃ and high thermo stability at 290-320℃. The water-casted SiO2-g-P(PEGMA)-b-P(12FMA) films obtained much rougher surface (125.3–178.4 nm) than THF-casted films (11.5–16.9 nm). Therefore, the water-casted surfaces exhibit obvious high water adsorption amount and hard adsorbed layer, but present loser adsorbed layer than THF-casted films. While, the introduction of P (12FMA) segments does not show obviously reduce in the protein-repelling adsorption of SiO2-g-P(PEGMA)-b-P(12FMA) films. The hydrophilic SDCNs of SiO2-g-P(PEGMA)-b-P(PEG) is prepared by PEGMA and poly(ethylene glycol) methacrylate (PEG). Their temperature sensitive behavior, pH response and surface properties as protein-resistance coatings are characterized. 220 nm core-shell nanoparticles are formed in water solution, which gained LCST at 60-77ËšC and good dispersion in water when pH>5.0. The introduction of P(PEG) segments could increase the protein-repelling adsorption of SiO2-g-P(PEGMA)-b-P(PEG) films. Therefore, these SDCNs are suggested to be used as protein resistance coatings.
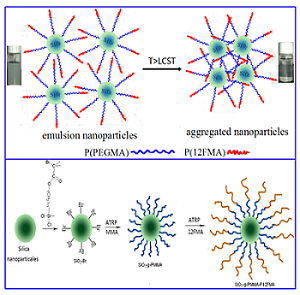
Figure.1: Synthesis of hydrophobic SiO2-g-PMMA-b-P12FMA and the aggregation of amphiphilic SiO2-g-P (PEGMA)-b-P (12FMA)
XIE Linghai
Nanjing University of Posts and Telecommunications, China
Title: The discovery of one-pot protocol to synthesize spiro[n]arenes
Time : 12:30-12:55

Biography:
XIE Linghai is a Professor of organic/polymer materials chemistry at Nanjing University of Posts and Telecommunications (NUPT). He obtained his PhD from Fudan University in June 2006. After this, he joined Nanjing University of Posts and Telecommunications (NUPT) and become a Leader of the Center for Molecular Systems and Organic Devices (CMSOD@IAM). He won the NSFC award for Excellent Young Scholar in 2013. His research activities focus on molecular installing technology (MIT), synergistically molecular attractor-repulsor theory (SMART), polygrid based nanopolymer wide-bandgap semiconductors, organic electrets for memories and memristors. He has published more than 150 papers in reputed journals and has been cited by more than 2000.
Abstract:
Four-element theory of matter-energy-information-consciousness (MEIC) guide human being to enter the era of consciousness (EOC) that will make service abundant extremely, resulting in the achievement of socialism and communism. Toward EOC, the surface-equal-device engineering is necessary to make the matter or objects smart that require the universe semiconductor materials. In order to make optoelectronic materials available easier, we always focus on the carbon-based organics for the investigation on the cycle of molecular systems and organic devices by means of the feature of diversity, flexibility and multiscale hierarchy. In the past decade, we discovered and established a facile one-pot protocol to synthesize the spiro[n]arenes and spirofluorenes with an orthogonal and cross-shaped configuration. The high-yield cascade tandem reaction makes spirofluorene xanthene (SFX) with low-cost, pot-atom-step economic (PASE) procedure. SFX-based chemistry has been conducted with various C-C or C-X coupling reaction to establish a diverse platform of bulky semiconductors for organic light-emitting diodes (OLEDs), transistor memory and perovskite solar cells, etc. Non-doped deep-blue SFX-based semiconductor was successfully prepared and adopted in OLEDs with external quantum efficiency (EQE) of 4.1%. Simultaneously, SFX-based host (SFXSPO) with a promising EQE of 22.5% was achieved in the corresponding thermally activated delayed fluorescence dives. Additionally, we have prepared SFX-based semiconductor (TTMeODPA-SFX) as hole-transporting material for perovskite solar cells (PSCs) and achieved improved power conversion efficiency of 12.94% and open-circuit photo-voltage of 1.00 V.
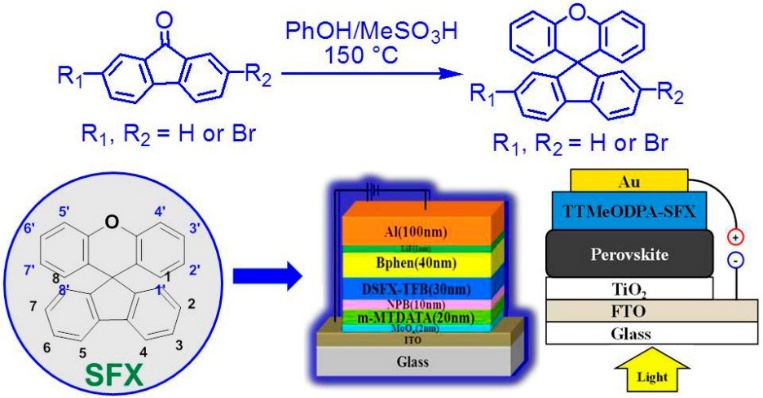
Fig.1: One-pot procedure of SFX-based semiconductors for OLEDs and solar cells.
Masao Takagaki
Kyoto University, Japan
Title: Concept of malignancy of brain tumors for drug design of boron neutron capture therapy
Time : 13:45-14:10

Biography:
Masao Takagaki has studied many subjects like Physics, Medicine, and Anthropology in Kyoto University. Currently, she is a Japanese Neurosurgeon who is investing Boron Neutron Capture Therapy for malignant brain tumors for more than 40 years. She has invested new boron/Gd compounds co-operating with many American Researchers.
Abstract:
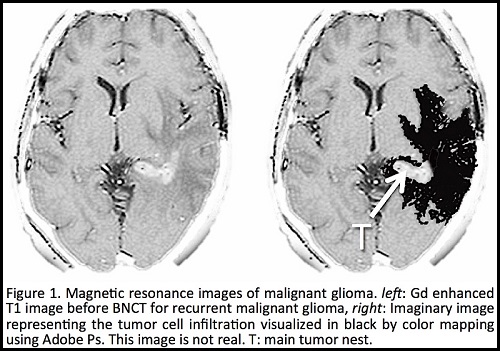
Boron Neutron Capture Therapy (BNCT) is a radiotherapy which combines biological targeting and high LET radiation. This type of therapy consists of the enrichment of tumors with 10B, and the successive irradiation of the target with low energy neutrons. This produces heavy ionizing particles that cause non-repairable damage to the cells. If 10B accumulates selectively in the tumor cells, high linear energy transfer (LET) radiation and tumor-selective radiation will not cause serious damage to the surrounding normal tissues. Previous phase-I studies shows that BNCT might be effective and safe in patients with inoperable, locally-advanced malignant glioma and head and neck cancers, even those that recur at previously irradiated sites. However, despite extensive efforts for over half century, it is difficult to ensure that the selective targeting of 10B will be successful. One can argue whether Locher's BNCT theory published in 1936 is feasible? We talk about the uncertainty and selectivity of boron targeting for malignant brain tumors (gliomas) and we presented about biological aspects for understanding of malignancy and intracranial tumor cells infiltration for organic and inorganic boron chemistry for BNCT targeting. From the viewpoint of boron chemistry, the conditions required for boron targeting are: (1) a low toxicity, (2) the ability to be held in a tumor and/or tumor cells selectively for a certain period of time, (3) to be rapidly excreted from the body system. These points are essential, but to achieve each of these at the same time is a very difficult task. Furthermore, we shortly discuss about the potential future problems of BNCT by reviewing an extensive number of recently published articles and author’s works regarding boron targeting for BNCT.
Yingju Xu
Merck & Co., Inc, USA
Title: Process development towards a green manufacturing route for letermovir exploiting novel asymmetric reactions
Time : 14:10-14:35

Biography:
Yingju Xu has attended Nanjing University, China, receiving her BA and MS in Organic Chemistry. She then conducted Graduate Research in the laboratory of Professor Soctt J Miller at Boston College, MA, earning her PhD in 2007. She joined the Department of Process Chemistry at Merck in 2007. She has 10 years of process development experience on various drug candidates. Her research experience at Merck focuses on discovery of innovative approaches to access drug candidate via low-cost and sustainable processes, design and development of robust green manufacturing processes for target drug candidates, as well as late stage process development to support filing and commercialization.
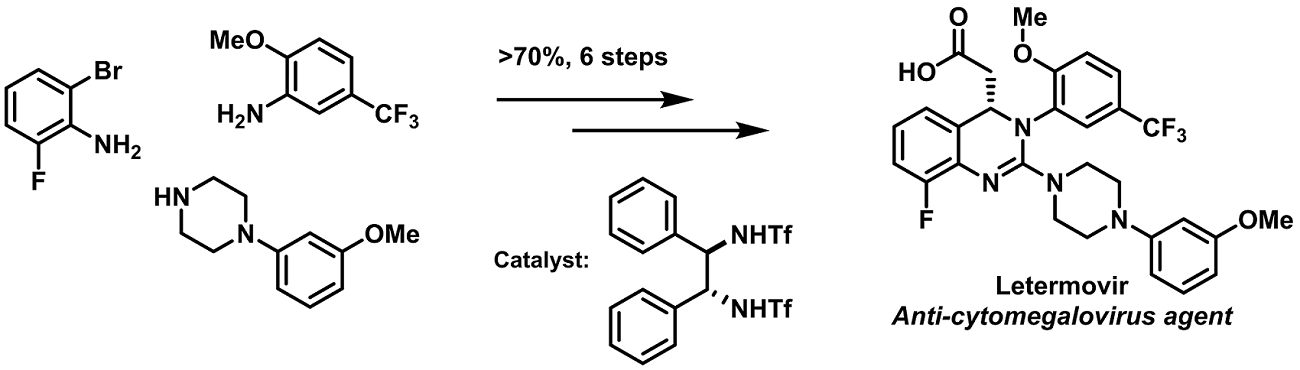
Abstract:
Merck aims to develop the best chemistry at time of regulatory filing, with the ultimate goal of the zero waste API manufacturing process. Achieving this ambitious goal is enabled by an innovative, green by design, development strategy, to progress from initial route design through to a fully optimized sustainable commercial manufacturing process. Letermovir is an antiviral drug currently in late-stage development for the treatment of cytomegalovirus infections. This presentation will describe key aspects of the synthesis development that led to an efficient, robust and green manufacturing process. A chemically stable and fully recyclable organo catalyst has been discovered to promote this novel asymmetric aza-Michael cyclization. The remainder of the synthesis has been fully optimized to reduce catalyst loading, minimize the number and amount of solvents, employ telescoped processing where possible, and to maximize atom-economy. Implementation, demonstration and validation of the new process were successfully completed in 2016. Compared to the benchmark process used for the generation of the initial phase III clinical supplies, this revolutionary new synthesis reduces PMI by 73%, increases the overall yield by more than 60%, and reduces raw material costs by 93%. Life-cycle assessment reveals that the new process reduces carbon footprint and water usage by 89% and 90%, respectively.
Nawarat Worauaychai
Ramkhamhaeng University, Thailand
Title: Corrosion behavior of TiC-Ni-5Mo2C cermet
Time : 14:35-15:00

Biography:
Nawarat Worauaychai has completed her PhD from Division of Materials Technology, School of Energy, Environment, and Materials, King Mongkut’s University of Technology Thonburi, Thailand. She is a lecturer at Department of Materials Technology, Ramkhamhaeng University. She has her expertise in powder metallurgy and corrosion study of alloys and composite materials. Most of her research ideas are mainly synthesize of new alloys and evaluation of mechanical properties and corrosion behavior.
Abstract:
Corrosion in TiC-Ni-5Mo2C cermet was studied in 0.3M H2SO4, 1M NaCl and 1M NaOH by means of anodic polarization method. Corrosion morphologies were then examined by SEM-EDS. The results showed that there are two ranges of passive region in acidic condition; whereas only one passive region was observed in alkaline solution. In case of chloride containing solution, passive region was hardly observed. The SEM micrographs revealed that nickel binder was a corrosion onset site in acidic and salt solution. In contrast to alkaline condition, the deterioration commenced at TiC. The driving force for corrosion susceptibility was the micro-galvanic cell formation between TiC and Nickel.
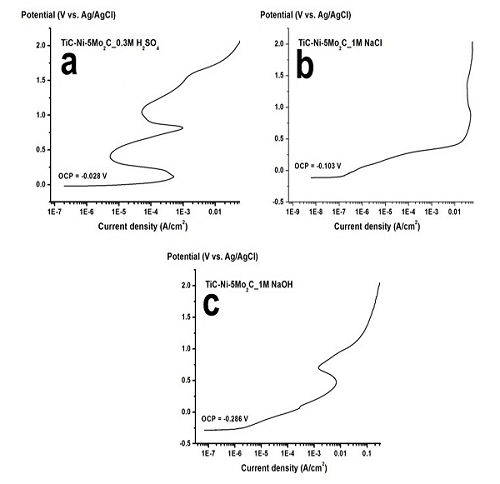
Figure 1: Potentiodynamic polarization curves of TiC-Ni-5Mo2C cermet in (a) 0.3M H2SO4 (b) 1M NaCl (c) 1M NaOH solution
Makanjuola Olatunji Mojeed
Federal University of Agriculture, Nigeria
Title: Effect of agrochemicals on metal speciation and their associated health risk in soil and vegetable species
Time : 15:00-15:25

Biography:
Abstract:
There is increasing global concern over the adverse effects of inorganic fertilizer and pesticides application on agricultural soils. Therefore, this study investigated the effect of agrochemical treatment on metal speciation and associated health risks in soil and vegetables species. 24 soil samples and nine varieties of vegetable species were collected from farm settlement in Ikorodu, Lagos, Nigeria and sequentially extracted using standard method. Extracts were analyzed for heavy metals using atomic absorption spectrophotometer, while health risk was assessed using United States Environmental Protection Agency (USEPA). The result reveals the redistribution pattern of heavy metals among the soil mineral components after chemical treatment. A comparison of metal concentrations in soil and vegetable species with standard set by USEPA shows that Cd level in the soil (13.54±1.21 mg/kg) and vegetables (0.83±0.05mg/kg) were above the critical permissible limit of 3.0mg/kg and 0.2mg/kg respectively. Based on soil pollution indices used for this study, it was deduced that the soil was practically not contaminated with all metals investigated in the soil except Cd which have very high contamination factor (CF), contamination degree (CD), pollution load index (PLI) and geo-accumulation index (Igeo). The cancer risk of heavy metals in the soil ranged from 1.02E−11 to 9.90E−10 and 6.70E−11 to 8.61E−09 for children and adult while that of vegetable species ranges from 6.03E−11 to 10.8E−10 and 3.70E−11 to 6.61E−09 for children and adult respectively. The level of cancer risk falls below the threshold values (10−4–10−6) which US Environmental Protection Agency considered as unacceptable risk.
Hyun-Joon Ha
Hankuk University of Foreign Studies, Korea
Title: Polyhydroxy alkaloids from aziridine
Time : 15:25-15:50

Biography:
Hyun-Joon Ha has obtained his BA from Seoul National University and PhD from Brown University. He has done his Postdoctoral studies from Stanford University (1987-1988). Then he came back to Korea and worked as a Senior Research Scientist at KIST. In the year 1991, he joined the faculty of the Chemistry department at Hankuk University of Foreign Studies, and is now a Professor of the Chemistry department at the same University. His research includes aziridine chemistry, synthetic methodology, lipase-mediated reactions, asymmetric synthesis with publications of more than 140 papers and 25 patents. He serves as an Associate Editor of Asian J. Org. Chem.
Abstract:
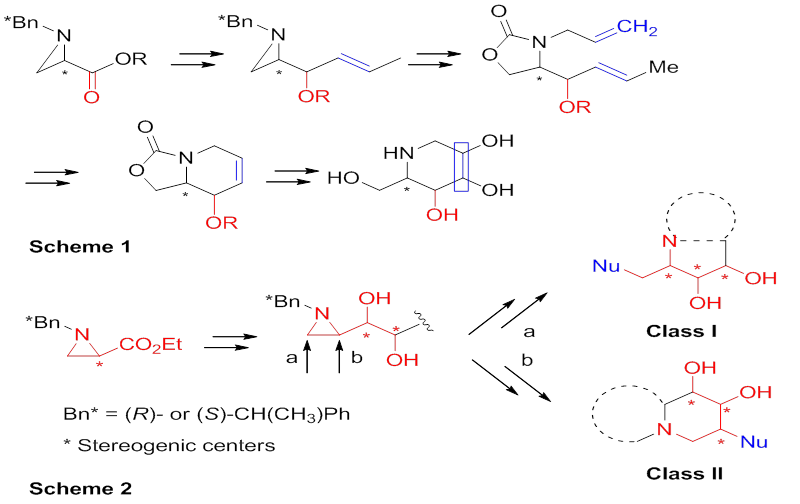
Alkaloids are nitrogen-containing natural products found in diverse organisms including fungi, plants, and animals. Among them, polyhydroxy alkaloids have a plethora of intriguing biological activities. Owing to their importance within the realm of medicinal and bioorganic chemistry, there is a rich literature detailing the attempts towards the synthesis of natural polyhydroxy alkaloids and their analogs. In most cases, those were prepared from uniquely functionalized starting materials with the assistance of a series of chemical transformations involving the construction of the carbon backbones followed by the stereoselective installation of hydroxyl groups. These synthetic strategies do not allow preparing multiple synthetic targets from a common synthetic intermediate. A chiral aziridine-2-carboxylate is a good starting material to elaborate polyhydroxy alkaloids, many of which were prepared in our laboratory in highly efficient manner with structural diversities. One of the typical examples is 1-deoxynojirimycin derived from hydroxyl functionalities using the common advanced synthetic intermediate via different hydroxylation methods. In additional polyhydroxy pyrrolidine alkaloids were also synthesized by branching two possible regiochemical pathways of ring-opening reactions of 2-substituted aziridine as a common synthetic intermediate providing an easy access to two different classes of compounds. Efficient regiochemistry-directed syntheses of monocyclic, bicyclic, and acyclic polyhydroxy alkaloids were achieved by taking different regiochemical pathways of aziridine ring-opening reactions of dihydroxylated aziridine as a common synthetic intermediate, which was readily available from commercial aziridine-2-carboxylate. Two possible regiochemical pathways of aziridine ring opening provided an easy access to two different classes of compounds from a common synthetic intermediate, called as regioselectivity-directed branches of 2-substituted aziridine. The applications of this synthetic strategy diversifing the stereoselective dihydroxylation and regioselective aziridine-ring opening as the key steps allowed the asymmetric synthesis of natural and unnatural polyhydroxy alkaloids including calyculin fragment C33-C37, 1,4-dideoxy-1,4-imino-L-ribitol and analogs of hyacinthacine, swainsonine, castanospermine and deoxynojirimycin.
Thomas E Müller
RWTH Aachen University, Germany
Title: Catalysis and life-cycle-analysis of current and future industrial routes to CO2 utilization
Time : 16:05-16:30

Biography:
Thomas E Müller is Lecturer at RWTH Aachen University, Germany. He has received a PhD in Chemistry (1995) from Imperial College London. After positions at University of Sussex and TU Munich and Visiting Professor position at NUS Singapore (2005) and University of Tokyo (2005), he accepted a position at Covestro in 2007. Since 2015 he has been concentrating on taking innovation to the chemical industry. He is Associate Editor of JCOU. His achievements include 80 papers and 75 patent applications, mainly in the fields of CO2 chemistry, catalyst development, and materials. His vision is to bridge the gap between fundamental research and industrial application.
Abstract:
The utilization of carbon dioxide (CO2) as an inexpensive and renewable C1 feedstock is of strategic importance for decreasing our dependence on fossil based raw materials (Ref. 1). In this context, the direct build-up of fuels, chemicals and polymers, at least partially, from CO2 is of particular interest. Various technologies are being discussed for implementation in the industry (Figure 1). The lecture will compare the current status of these technologies, the changes effected upon introducing CO2 as feedstock and evaluate the CO2 reduction potential based on the currently available life-cycle-analyses. One recently commercialized process that involves the direct use of carbon dioxide as a feedstock is the production of organic carbonates by reaction of CO2 with epoxides (Ref. 2-4). Indirect routes for CO2 utilization are of equal relevance. Formaldehyde, commercially attained by partial combustion of methanol that, in a future economy, may be obtained from CO2, is a promising feedstock. Thus, oligomeric poly (acetalester) is generated readily from formaldehyde. Such poly (acetalester) has intriguing properties rendering them inspiring compounds for fuel and polymer applications. Motivated by the goal of expanding the CO2 technologies to further application fields, it seems essential to unravel highly effective strategies for activating the carbon dioxide molecule. The energy needed to overcome the thermodynamically low energy level of CO2 is thereby a crucial factor. Only when the energy level of the resulting intermediates is appropriate, the subsequent reaction of the activated CO2 molecule with the co-reagents will take place. The right choice of the catalyst is thus the key to CO2 conversion and directs the reaction pathway to the desired target products. The lecture concludes with discussing some of the most intriguing approaches for activating CO2.
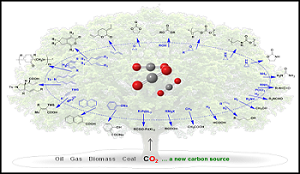
Figure.1: The utilization of carbon dioxide (CO2) as an inexpensive, renewable and abundant C1 feedstock is of strategic importance for decreasing our dependence on petroleum-derived raw materials.
Marco Giulio Rigamonti
Politecnico di Milano, Italy
Title: Stereoselective synthesis of hernandulcin, peroxylippidulcine A, lippidulcines A, B and C and taste evaluation
Time : 16:30-16:55

Biography:
Marco Giulio Rigamonti studied chemical engineering at Polytechnique of Milan and graduated with top of marks under the supervision of prof. F. Gatti in 2014. Currently he is pursuing a PhD in Polytechnique de Montreal with prof. G. Patience, working on spray drying, computational analysis and scale up for Li-ion battery manufacturing and catalyst for fluidized bed reactors.
Abstract:
Sucrose abuse is strongly associated to many undesirable health effects. Artificial sweeteners are a popular alternative but many raise questions regarding their safety. The Mexican plant Lippia dulcis contains trace amounts of (+)-hernandulcin, a compound so sweet – 1000 times more that sucrose–that few leaves are enough to sweeten a cup of tea. Recent studies revealed that the plant also produces several derivatives of this molecule: The peroxylippidulcines and the lippidulcines A, B and C. However, these sesquiterpenes have been isolated in such a small amount that it has not been possible to assess their taste. A multigram scale-up and optimization for the synthetic route of (+)-hernandulcin, allowed us to accomplish the first stereoselective synthesis of lippidulcines A, B and C. With modified version of the Kornblum–DeLaMare rearrangement, and a highly regioselective and stereoselective ketone reduction with the MeCBS reagent we synthesized the compounds and confirmed the previously assigned absolute configuration. The taste evaluations indicate that lippidulcine A is a cooling agent with a mint after taste, while none of these sesquiterpenes are sweet. Indeed, the insertion of a hydroxy group on the side chain of hernadulcin annuls its intense sweetness.
Ziwei Gao
Shaanxi Normal University, China
Title: Multi-functional N, O donors for Cu catalyzed Csp2-Csp cross-coupling reaction
Time : 16:55-17:20

Biography:
Abstract:
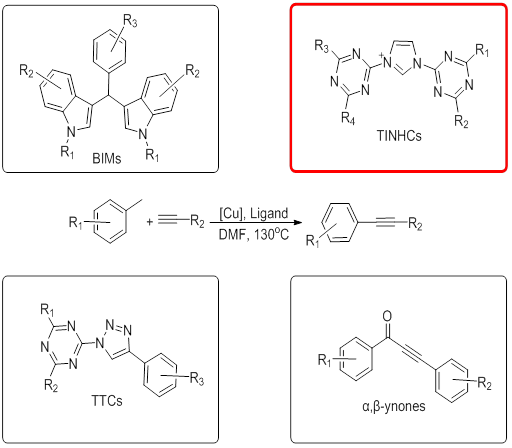
The Sonogashira reaction is one of the most important and widely used Csp2–Csp bond formation reactions in organic synthesis, frequently employed in the synthesis of natural products, biologically active molecules, heterocycles and molecular nanostructures. Various attempts have been made to achieve the Sonogashira reaction in the absence of the expensive noble metal palladium, and copper has proved an excellent alternative. With intensive study of the copper-based catalyst system, it is found that the coupling efficiency is greatly enhanced by 2e electron donor ligands. Since Miura and co-workers introduced the CuI/PPh3-catalyzed cross-couplings of aryl iodides and aryl acetylenes, hard donors as green and efficient promoters have dominated the copper-catalyzed coupling reactions instead of the classic phosphine ligands. Recently, we developed four kinds of multi-functional ligands which significantly enhanced the activity of low-mol% (1-5%) copper precatalysts. N, O donors ligands of bisindoles (BIMs), Triazine-Triazole conjugates (TTCs), Triazine-imidazole N-heterocyclic carbenes (TINHCs) and ynones have ensured the robust and straight forward cu catalyzed cross-coupling of Csp2-Csp bond, making this an indispensable methodology for the synthesis of complex molecular architectures of C≡C. These ligands inherit both the stability and activity of triazines and aromatic system, which were easily synthesized by multi-step nucleophilic substitution reaction.
Zhongyi Ma
Chinese Academy of Sciences, China
Title: Comparative study of CO adsorption on zirconia polymorphs by diffuse reflectance FT-IR and transmission FT-IR spectroscopy
Time : 17:20-17:45

Biography:
Zhongyi Ma is an associate professor at Institute of Coal Chemistry, Chinese Academy of Science. He got his Bachelor degree at Qufu Normal University in 2000 and his PhD at Institute of Coal Chemistry, Chinese Academy of Science in 2005. He has 10 years of industrial experience in the petrochemical industry. He worked as Postdoctoral at South Dakota State University during 2011-2012. He is working as Associate Professor in Institute of Coal Chemistry. His research fields include Fischer-Tropsch synthesis, biomass conversion.
Abstract:
Interests in the surface properties of zirconia aroused a steady interest over many years because this solid exhibited catalytic behavior in dehydration, hydrogenation, and hydrogen exchange reactions. A great deal of work has been devoted to the study of zirconia, as both a catalyst and a support for metal catalysts. In this study the interactions of CO with amorphous zirconia (am-ZrO2), monoclinic zirconia (ZrO2), and tetragonal zirconia (t-ZrO2) were investigated by diffuse reflectance FT-IR spectroscopy and transmission FT-IR to determine the influence of zirconia polymorphs on CO adsorption and conversion. Moreover, the effects of dehydroxylation degree of different zirconia polymorphs on CO adsorption at ambient temperature was also surveyed at different thermal vacuum treatments were observed by transmission FT-IR. The results showed that zirconia polymorphs exerted a great influence on CO adsorption, the formation and conversion of surface intermediates, and the active sites for CO adsorption varied with the dehydroxylation degree of zirconia polymorphs. CO bands were detected on am-ZrO2 and m-ZrO2 samples either by CO adsorption at high temperature or CO adsorbed on dehydroxylated samples. The former was achieved by formation of formate species at high temperature via CO reacting with surface hydroxyl. The latter achievement was accomplished by the thermal dehydroxylation to decrease the surface coordinative water and hydroxyl. While in the case of t-ZrO2 sample, CO band was only created by the formation of bicarbonate species at high temperature to produce the cationic sites. The active sits for CO adsorption were created by CO reacting with surface hydroxyls at elevated temperature in the case of DRIFT spectra, while the active sites were produced by the thermal dehydroxylation in transmission FT-IR spectra.
A) 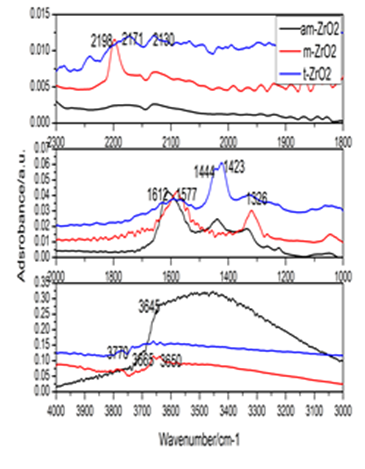
 B)
B)
Fig.1: FT-IR of CO adsorption on ZrO2 polymorphs. A: DRIFT spectra; B: Difference transmission infrared spectra
Wei Zhang
Chinese Academy of Sciences, China
Title: The synthesization of SAPO-11 and its catalytic performance for the alkylation of naphthalene
Time : 17:45-18:10

Biography:
Wei Zhang is an Associate Professor at Institute of Coal Chemistry, Chinese Academy of Science. He got his Bachelor degree at Taiyuan University of Technology in 1999 and his PhD at Institute of Coal Chemistry, Chinese Academy of Science in 2010. From 2010 till now, he has been working as Assistant Professor in Institute of Coal Chemistry. His research fields include Fischer-Tropsch synthesis, biomass conversion.
Abstract:
The SAPO-11 zeolite is a member of the silico-aluminophosphate (SAPO-n) family which was first synthesized by Union Carbide Corporation. It has novel pore structures and exhibits milder acidity due to the presence of phosphorus. The pore size of SAPO-11 was more than 0.60nm, which can sieve effectively the products of the methylation of naphthalene. In this study, SAPO-11 zeolites were successfully synthesized by using three different templates (diethylamine (DEA), di-n-propylamine (DPA) and di-iso-propylamine (DIPA)) under hydrothermal conditions. The structures and acidities of SAPO-11 were characterized by means of XRD, BET and NH3-TPD. XRD results indicated that the directing effect of different templates for AEL structure decreased in the order of DPA >DEA>DIPA. N2 adsorption-desorption results showed that SAPO-11(DPA, 1.2) possesses higher surface area and larger pore volume than other samples, which corresponds to the higher crystallinity. This is in agreement with the results of the XRD.
The alkylation of naphthalene (NAPH) with methanol (ME) on the SAPO-11 zeolite was investigated. 1, 3, 5-trimethylbenzene (TMB) was used as solvent. The effects of the reactant mol ratio, the reaction temperature and the weight hourly space velocity (WHSV) on the conversion of NAPH and the selectivity to 2, 6-dimethylnaphthalene (2, 6-DMN) was studied. The results showed that the optimum reaction conditions are as follows: 0.1 MPa, 425℃, n(NAPH)∶n(ME)∶n(TMB)=1∶5∶3.5, WHSV 0.06 h−1.
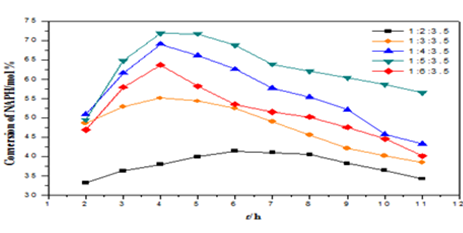
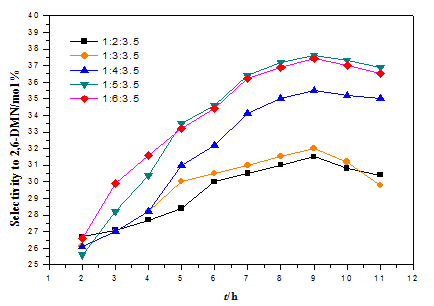
Fig.1: The effect of reactant mol ratio on the conversion of NAPH and the selectivity to 2, 6-DMN
Abdolreza Hajipour
Isfahan University of Technology, Iran
Title: Co nanoparticles as efficient catalysis in homo- and hetero coupling reaction
Time : 18:10-18:35

Biography:
Abstract:
In recent years, among the first-row transition metal catalysts, cobalt catalyst has attracted much attention in organic synthesis because it is readily available, non-toxic, low-cost, and stable in air and exhibits high catalytic activity. However, to the best of our knowledge aryl halide–heteroatom cross-couplings using cobalt salts are rare, catalyst application of cobalt salts combined with ligands have explored as a suitable catalyst for construction of C–N and C–O bonds via dehalogenative paths. In a continuation of our recent investigations in developing efficient catalysis and their application in organic synthesis, herein, we would like to report new green and economical methods for the synthesis of organic compounds using cobalt nanoparticles (Co-NP) as green, efficient and non-toxic catalyst.
Wen-Yong Lai
Nanjing University of Posts and Telecommunications, China
Title: π-extended star-shaped polycyclic aromatic hydrocarbons: Synthesis, self-assembly and facile-tunable emissive properties

Biography:
Wen Yong Lai is a Full Professor at the Nanjing University of Posts and Telecommunications. He received his PhD from Fudan University in 2007. He then joined the Key Laboratory for Organic Electronics & Information Displays and Institute of Advanced Materials, Nanjing University of Posts & Telecommunications. His research mainly focuses on the design, synthesis and application of organic and polymer optoelectronic materials for organic/plastic electronics. He is also interested in the exploration of novel materials and processes for printed electronics.
Abstract:
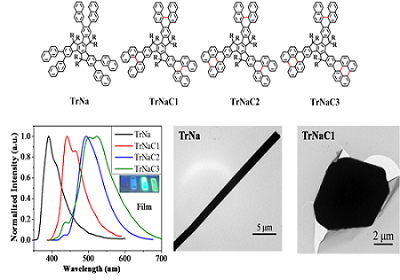
A novel set of star-shaped polycyclic aromatic hydrocarbons (PAHs) based on naphthalene-fused truxenes, named as TrNaCn (n=1-4), were synthesized and characterized. Oxidative cyclodehydrogenation was carried out following the microwave-assisted six-fold Suzuki coupling reaction. It is worthwhile to mention that multiple dehydrocyclization samples can be isolated effectively in one reaction, suggesting that the oxidative cyclodehydrogenation was a stepwise ring-closed process. Thermal, optical, and electrochemical properties, and self-assembly behaviors of the resulting oxidized samples were investigated to understand the impact of the ring-fused process on the properties of star-shaped PAHs. Distinct bathochromic shift of the absorption maxima λmax reveals that the molecular conjugation extends with the stepwise ring closed reaction. The optical band gap energy of these PAHs varied significantly with increasing the fused rings, resulting in facile-tunable emissive properties for the resultant star-shaped PAHs. Interestingly, with the generation of perylene analogue rigid arms, TrNaC2 and TrNaC3 showed significant enhancement of photoluminescence quantum yields (PLQYs) in solution (η=0.65 and 0.66, respectively) in comparison with those of TrNa and TrNaC1 (η=0.08 and 0.16, respectively). With strong intermolecular interactions, the precursor TrNa is found to be able to self-assemble into rod-like microcrystals which can be facilely identified by naked eyes, while TrNaC1 self-assembles into nanosheets once the naphthalene rings are fused. This study offers a unique platform to gain further insights and better understanding into the photophysical and self-assembly properties of π-extended star-shaped PAHs.

Biography:
Akhilesh Kumar Verma obtained his PhD degree in 2000 from the Department of Chemistry, University of Delhi, India. He was a postdoctoral Fellow with Professor Alan R Katritzky at the University of Florida, Gainesville, USA, from 2001 to 2002, and with Prof. Richard C Larock at Iowa State University of Science and Technology, Ames, Iowa, USA, from June 28, 2007 to August 31, 2008. He started his career as a Lecturer in 1998 at the Dr. B R Ambedkar Center for Biomedical Research at the University of Delhi, India. He joined the Department of Chemistry University of Delhi in January 2009 as a Reader. He was appointed as Associate professor in 2010 and since 2013 he is Full Professor in the department. His research interests include developing new synthetic methodologies; heterocyclic synthesis using alkynes and designing of benzotriazole based novel ligands for copper and palladium-catalyzed coupling reactions.
Abstract:
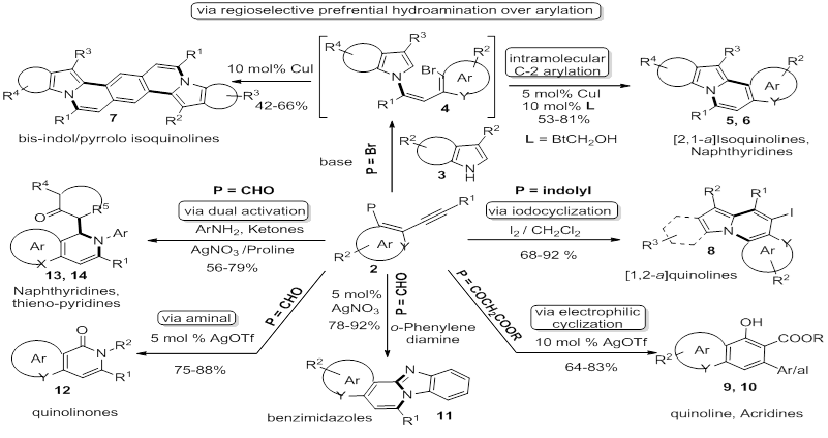
Synthesis of small heterocyclic molecules in terms of selectivity, operational simplicity, functional group tolerance and environmental sustainability are in constant demand as majority of drugs; drug-like compounds contain hetero atom at their core. In continuation of our interest in the synthesis of heterocycles using alkynes, we have successfully engineered the synthesis of variety of biologically important heterocyclic scaffolds using electrophilic cyclization/hydroamination/and alkyne annulations. In this presentation the author would like to discuss about recent results in this chemistry.
Isaiah Ramaite
University of Venda, South Africa
Title: Isolation, characterization and biological evaluation of Terminalia serecia metabolites

Biography:
Isaiah DI Ramaite has done his PhD in Physical Organic Chemistry from Rhodes University. He has been lecturing at higher education for the past 20 years. His main research interest is organic synthetic chemistry of heterocyclic compounds and also natural products chemistry of plants that are currently being used by traditional healers in Limpopo Province, South Africa. He is also interested in chemical education for both high school learners and undergraduate university students.
Abstract:
The Limpopo province has a rich biodiversity of a wide range of plants which are currently being exploited by traditional healers for the treatment of various diseases. As part of an ongoing research programme in natural products at the University of Venda, we have been interested on Terminalia sericea Burch ex. DC. Ethno medicinal information revealed that the fruit, leaves, stem bark and roots of T. sericea are commonly used for the treatment of coughs, skin infections, diabetes, diarrhea, and gonorrhea. Anolignan B and termilignan B isolated from the root have been reported to possess antibacterial activity against Bacillus subtilis, Staphylococcus aureus, Escherichia coli and Klebsiella pneumoniae. The aim of our study was to isolate, purify and evaluate the biological activity of various fractions and pure compounds. Various spectroscopic techniques such as 1D and 2D, HRMS and IR were used to elucidate the structures of pure compounds. This study has shown that the antibacterial constituents of T. sericea root cannot be limited to compounds with lignan structure which have been isolated previously from fractions obtained from mixture of hexane and ethyl acetate using silica gel. The presentation will focus on the results of antibacterial activity against diarrhea pathogens and skin infection organism, and isolated compounds responsible for these activities including their structural elucidation
Pallavi Mangesh Patil
P E Society’s Modern College of Pharmacy, India
Title: Stability-indicating HPTLC method for simultaneous determination of ketoprofen, methyl paraben and propyl paraben in gel formulation

Biography:
Abstract:
Aim: A novel and quick HPTLC-densitometry method was developed for the simultaneous determination of ketoprofen, methyl paraben and propyl paraben.
Methods: Chromatographic separation of the drugs was performed on precoated silica gel 60F254 Merck plates using toluene: ethyl acetate: glacial acetic acid (6.5:2.5:1.0 v/v/v) as a mobile phase. A TLC scanner set at 265 nm was used of ketoprofen, methyl paraben, propyl paraben respectively were validated according to ICH guidelines. Forced degradation conditions of hydrolysis (neutral, acidic and alkaline), oxidation, photolysis and thermal stress as suggested in the ICH guideline Q1A (R2).
Results: The three drugs were satisfactorily resolved with Rf values of 0.33_0.05, 0.54_ 0.05, 0.71_0.05 for ketoprofen, methyl paraben, and propyl paraben respectively. Calibration curves were polynomial in the range 200e1000 ng/band, 200e1500 ng/band, 100-600 ng/band, for Ketoprofen, Methyl Paraben, and Propyl Paraben respectively. Correlation coefficient (r) values were 0.9917, 0.9927, 0.9906 Ketoprofen, Methyl Paraben, Propyl Paraben respectively. The percentage recovery ranges from 99% to 101%.
Conclusion: A low relative standard deviation (<2%) was found for both precision and robustness study showing that the proposed method was precise and robust. The method had an accuracy of 99.95%, 99.85% and 100.07 of ketoprofen, methyl paraben, propyl paraben respectively were validated according to ICH guidelines. The drug showed instability in oxide, heat and UV light, while it remained stable in neutral conditions.
Adeyemi Olufemi
Olabisi Onabanjo University, Nigeria
Title: Biosorption of cadmium and lead ions from aqueous solution of cashew (Anacardium occidentale) leaf

Biography:
Abstract:
The potential of using cashew (Anarcardium occidentale) leaf to remove Pb (II) and Cd (II) ions from aqueous solution was investigated. The influence of pH, contact time and initial metal ion concentration and temperature were studied using batch experiment. The biosorption of the metal ions was found to be pH dependent. Analysis of the FT-IR spectra showed the presence of ionizable functional groups (C=O, O-H) which were able to interact with cations and thus served as the active sites for the removal of positively charged Pb (II) and Cd (II) ions from solution. Thermodynamic parameters such as the free energy change (ΔG), ΔH and ΔS were evaluated it was found that the sorption process was feasible, spontaneous and endothermic.
Yinghuai Zhu
Agency for Science, Technology and Research (A*STAR), Singapore
Title: Advanced developments in boron neutron capture and therapy (BNCT) and related nano catalysis

Biography:
Abstract:
Boron neutron capture therapy (BNCT) continues attracting wide interesting due to its high performance in cancer treatment and significant progress in neutron beam study. We have developed the low toxic and high efficient BNCT agents, which demonstrated high selectivity of tumor to normal tissues. In this presentation, we deliver the recent progresses in BNCT research and nano catalysis technology that is exceedingly useful in boron chemistry.
- Sessions/ Tracks:
Bioorganic and Biochemistry | Advance Trends in Organic Chemistry | Inorganic and Bioinorganic Catalysis | Physical Organic Chemistry | Medicinal Chemistry, Drug Synthesis | Industrial Inorganic Chemistry | Inorganic Materials and Nanoparticles | Stereochemistry of Organic Compounds | Green Chemistry and Sustainable Technology | Organic Chemical Engineering

Chair
Bhanu P S Chauhan
William Paterson University, USA

Co-Chair
Rocio Gamez-Montano
Universidad de Guanajuato, Mexico
Session Introduction
Thornthan Sawangwan
Ramkhamhaeng University, Thailand
Title: Novel maltooligosaccharide from biosynthesis
Time : 11:45-12:10

Biography:
Thornthan Sawangwan has her expertise in biosynthesis and application of microbial and bioactive compound to functional food. Her research idea interest is mainly on prebiotic compound synthesized by bioconversion from economical substrate and agroindustry residue. Her laboratory skills are Microbiology, Enzymology, chromatography and spectrophotometry techniques. She has experience of many years in Research and Teaching in Ramkhamhaeng University, Bangkok, Thailand.
Abstract:
This research focuses mainly on synthesis of maltooligosaccharide (MOS) by using biocatalytic method. Two commercial cyclodextrin glucosyltransferases (CGTase), Toruzyme®, AMANO, and whole cell of glucosyltransferase (GTase) obtained from a culture of Eschericia coli with Thermotoga maritime_GTase strain (Tm_GTase) are used for MOS synthesis. Before start to synthesis experiment, all biocatalyst are determined of protein concentration by using Bradford technique. The reaction mixtures for MOS synthesis are comprised with soluble starch as a donor, maltose and glucose as acceptor in different ratios (1:0, 4:1, 1.5:1, 1:1, 1:1.5, 1:4 and 0:1 w/w) in 10 mM sodium citrate buffer pH 5.5+0.15 mM CaCl2 compared with the reaction in distilled water (pH 6.3) at 60°C and 85°C incubation temperature. After the appropriate incubation times, the product mixtures are stopped for the reactions by adding 0.4 N NaOH, centrifuged at 13,200 rpm for 15 minutes then analyzed by High Performance Liquid Chromatography (HPLC); HPX-87H column and ion chromatography (Dionex); Carbopac PA10 column. The considering formation of MOS products from 3 different biocatalytic processes are short chain MOS; maltotetraose (G4) and maltotriose (G3) as shown in the chromatograms of HPLC and Dionex determination. From the results, the noticeable synthesis of MOS products are in the condition of maltose as an acceptor in 10 mM sodium citrate buffer pH 5.5+0.15 mM CaCl2 using Toruzyme® and AMANO at 85°C incubation temperature. These conditions show higher MOS yield compare with using Tm_GTase strain as biocatalyst. In conclusion, CGTase from Toruzyme® and AMANO can use for synthesis MOS products (especially maltotetraose and maltotriose) which have more interest for food industries in order to apply for functional food as prebiotic compounds.
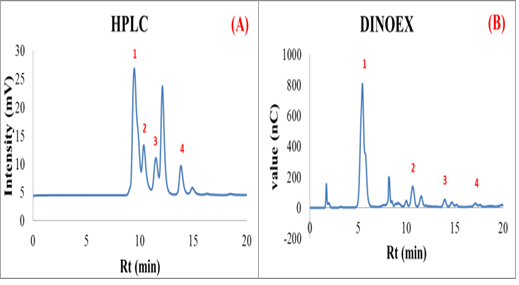
Figure 1: The chromatogram of product mixture from MOS biosynthesis (A) HPLC chromatogram; 1- maltotetraose, 2- maltotriose, 3- maltose, 4- glucose and (B) Dionex chromatogram; 1- glucose, 2- maltose, 3- maltotriose and 4- maltotetraose.
Yoshiyuki Kageyama
Hokkaido University, Japan
Title: Design and creation of autonomous dynamics in self-assemblies
Time : 12:10-12:35

Biography:
Yoshiyuki Kageyama has obtained his PhD from the University of Tokyo in 2006 under the direction of Prof. Shigeru Murata. He continued his study of the construction of biomimetic supra molecular systems based on organic chemistry as a Postdoc in Prof. Tadashi Sugawara's group at the University of Tokyo. Next he moved to the Faculty of Pharmaceutical Science at Tokyo University of Science as a Postdoc and studied organic photochemistry and molecular-recognition chemistry in aqueous media under the direction of Prof. Shin Aoki. In 2009, he became an Assistant Professor at Laboratory for Condensed Matter Chemistry at Hokkaido University. He worked at Japan Science and Technology Agency as a PRESTO researcher (2013-2017). His specialty is in physical organic chemistry, especially the kinetic study of organic reactions and supra molecular chemistry.
Abstract:
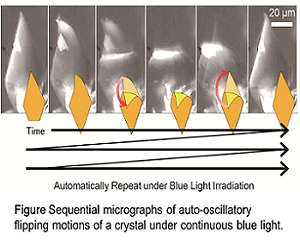
My research field in organic chemistry creates collective and hierarchical movements of molecules in synthetic chemistry. The ultimate goal is to create a reaction system that imitates the living body, that is, create artificial life. The specialty of the chemical process of living organisms lies in where individual reactions progress in a specific and efficient manner and reactions are synchronized to realize continuous molecular group behavior. The spatiotemporal behavior of this biological system is dissipative self-organization, which is far apart from the properties of common substances that apparently stop reactions and exercise at steady state. From the view point of mimetics of the characteristics of such life, synthetic chemical research to create molecular assemblies that continue some dynamic action under non-equilibrium where energy or chemical substances flow in, have been underwent by our group. Herein, we will talk two results of our research. One is auto-catalytic vesicular self-reproduction, and another is auto-oscillatory flipping motion of organic crystal. These two fundamental results will contribute to create more sophisticated chemical systems having autonomous properties.
Toshiyuki Moriuchi
Osaka University, Japan
Title: Vanadium-catalyzed bromination reaction with molecular oxygen
Time : 12:35-13:00

Biography:
Toshiyuki Moriuchi received his bachelor’s degree in 1991 and his doctoral degree in 1995 under the supervision of Professor Toshikazu Hirao, both from Osaka University. He became Assistant Professor at Osaka University and was a postdoctoral fellow at California Institute of Technology with Professor Jacqueline K. Barton (1996–1997). Dr. Moriuchi was promoted to Associate Professor in 2004. His current research interests focus on the development of novel artificial bioconjugated systems based on self-organization of biomolecules and redox-active p-conjugated systems for functionalized catalysts and materials. He received the Inoue Research Award for Young Scientists in 1997 and HGCS Japan Award of Excellence 2011 in 2012.
Abstract:
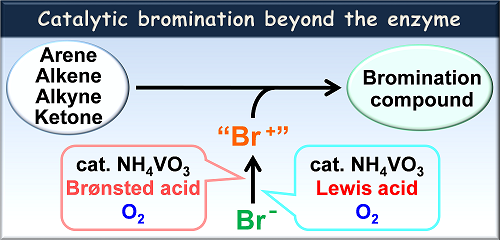
Bromination of organic compounds is one of the most fundamental reactions in organic synthesis, providing important precursors and substrates in various coupling reactions. Hazardous and toxic elemental bromine is utilized in a conventional bromination reaction. To avoid the use of bromine, considerable efforts have been devoted to develop an environmentally-favorable bromination method with a bromide ion as a bromide source. Vanadium bromoperoxidase catalyzes two-electron oxidation of a bromide ion in the presence of hydrogen peroxide, affording a bromonium cation-like species, which induces the bromination of organic compounds. So, the bromination reaction mimicking a catalytic activity of vanadium bromoperoxidase has attracted much attention. These methodologies, however, require a stoichiometric amount of a strong oxidant to generate a bromonium-like species. A more practical catalytic bromination reaction system without use of hazardous reagents is to be developed. From the view point of green chemistry perspective, molecular oxygen is considered as an ideal oxidant. We embarked upon the development of an environmentally-favorable catalytic system for selective bromination of a wide range of substrates. In this presentation, versatile and practical catalytic bromination systems by the combination of a commercially available inexpensive vanadium catalyst and a Brønsted acid or a Lewis acid under molecular oxygen will be described.
Anil K Tripathi
Indian Institute of Integrative Medicine (CSIR), India
Title: Synthesis of 7-(2-substituted-phenylthiazolidinyl)-benzopyran-2-one derivatives
Time : 13:50-14:15

Biography:
Anil K Tripathi has completed his PhD from Barkatullah, Vishwavidalaya, Bhopal. He is a Senior Scientist in IIIM Jammu (CSIR-Govt. of India), a premier Scientific Organization. He has published more than 25 papers in reputed journals and has been serving as Reviewer of reputed peer reviewed International journals.
Abstract:
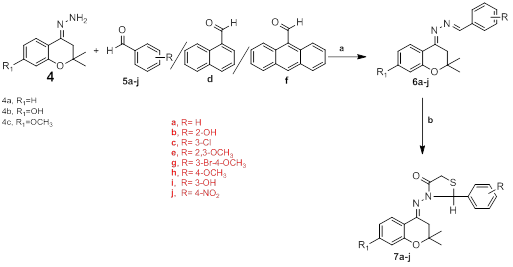
The molecular exploitation of promising lead compounds is still a major line of approach to develop new drugs. It involves an effort to combine the separate pharmacophoric groups of similar activity into one compound, thus making structural changes in the biological activity. Thiazolidinone ring present in a large number of biologically active molecules of different pharmacological classes exhibited different activities. The historical importance of thiazolidine derivative was emphasized during the period 1941–1945, i.e. the development of penicillin which shows the presence of thiazoline ring. The discovery of benzopyran like derivatives as therapeutic agents in early 1820’s was the beginning of the chroman related drug development. The chroman derivatives have a relatively broad spectrum with high activity proï¬le against various bacteria and fungi. Due to the diversiï¬ed nature of chroman and thiazolidinone, which render them to be useful substances in drug researches. In continuation of our search for novel biologically active benzopyran derivatives, in this paper we report the synthesis and antibacterial/anti-cancer/anti-inflammatory activity of a novel series of Schiff bases of 2, 2-dimethyl-4-hydrazone chromanone and its derivatives 4-substituted phenylthiazolidinyl chroman derivatives. The synthetic strategy is delineated in Scheme 1. Numerous keto compounds reacted directly with hydrazine hydrate in ethanol to obtain substituted hydrozones which was further treated with various formyl derivatives in absolute alcohol using catalytic amounts of acetic anhydride to afford substituted shiff’s bases. To conclude this particular reaction sequence finally, treated the shiff’s bases with thioglycolic acid in dioxan in presence of zinc chloride we obtained substituted phenylthiazolidinyl chroman derivatives (7a-j). All the synthesized compounds were purified by column chromatography before characterization by spectroscopic methods.
Adeyemi Olufemi O
Olabisi Onabanjo University, Nigeria
Title: Biosorption of cadmium and lead ions from aqueous solution of cashew (Anacardium occidentale) leaf
Time : 14:15-14:40

Biography:
Abstract:
The potential of using cashew (Anarcardium occidentale) leaf to remove Pb (II) and Cd (II) ions from aqueous solution was investigated. The influence of pH, contact time and initial metal ion concentration and temperature were studied using batch experiment. The biosorption of the metal ions was found to be pH dependent. Analysis of the FT-IR spectra showed the presence of ionizable functional groups (C=O, O-H) which were able to interact with cations and thus served as the active sites for the removal of positively charged Pb (II) and Cd (II) ions from solution. Thermodynamic parameters such as the free energy change (ΔG), ΔH and ΔS were evaluated it was found that the sorption process was feasible, spontaneous and endothermic.
Marco G Rigamonti
Polytechnic University of Milan, Canada
Title: Vanadium pyrophosphate catalyst: Core-shell morphology and attrition resistance
Time : 14:40-15:05

Biography:
Marco G Rigamonti has studied Chemical Engineering at Polytechnique of Milan and graduated with top of marks under the supervision of prof. F Gatti in 2014. Currently he is pursuing a PhD in Polytechnique de Montreal with prof. G Patience, working on spray drying, computational analysis and scale up for Li-ion battery manufacturing and catalyst for fluidized bed reactors.
Abstract:
Exothermic heterogeneous catalytic reactors are difficult to design when facing hotspots, radial and axial concentration and temperature gradients and run-away. Cylindrical pellets are the typical form for catalysts in fixed beds and the resulting long internal diffusion lengths limits the kinetics. Fluidized bed minimizes diffusional limitations with catalyst powders of 70 µm in diameter. Jet velocities on the order of 50 m s-1 mix the gas and solids thoroughly, resulting in high heat and mass transfer rates. However, the catalyst must withstand severe mechanical stresses induced by the jets. Creating attrition resistant porous micro-spheres is fundamental to compete economically with fixed bed reactor technology. Here, we synthesize vanadium pyrophosphate catalyst (VPP) as a template material to examine the effect of binders and spray drying conditions on attrition resistance and particle shape, size and porosity. A wet media mill (Netzsch mini-fer) ground commercial VPP precursor to a suspension of 7 µm and 0.5 µm primary particles in water. We tested several combinations of binders and additives including colloidal silica, polyvinyl alcohol, NaOH or H3PO4. A two fluid nozzle atomized the slurry inside a 0.8 m diameter drying chamber at 250ºC. EDS elemental mapping images demonstrate that the silica migrates to the surface during the drying process and forms a shell. Longer milling time achieves a smaller primary particle and the ASTM attrition test confirms a higher attrition resistance.

Sara Arafeh
Marquette University, USA
Title: Quantitative detection of the conformational transitions between open and closed forms of cytochrome P450 oxidoreductase (CYPOR) at the membrane surface in different functional states
Time : 15:05-15:30

Biography:
Sara Arafeh is PhD student in Biophysical Chemistry at Marquette University, Milwaukee, USA.
Abstract:
Cytochrome P450 and monooxgenases require a supply of electrons to catalyze the synthesis of steroid hormones, fatty acids and prostaglandin hormone. Cytochrome P450 oxidoreductase (CYPOR), a membrane bound enzyme, provides these electrons in its open conformation. CYPOR has two cytosolic domains (FAD domain and FMN domain) and an N-terminal in the membrane (Figure 1). In its open conformation, electrons flow from NADPH, FAD, and finally to FMN where cytochrome P450 picks up these electrons. In the closed conformation, cytochrome P450 does not bind to the FMN domain to take the electrons (Figure 2). It was found that when the cytosolic domains are isolated, CYPOR cannot bind to cytochrome P450. This suggested that the membrane environment is important for CYPOR function. Studies on the open/closed conformations of the full-length CYPOR have never been done, so this project takes the initiative to better understand the function of CYPOR in its full length. Here, we determine the distance between specific sites in the FAD and FMN binding domains in CYPOR by Forster Resonance Energy Transfer (FRET) and Ultrafast TA spectroscopy with and without NADPH. The approach to determine these distances will rely on labeling these sites with red and infrared fluorophores. Mimic membrane attachment is done by inserting CYPOR in lipid nanodiscs. By determining the distances between the donor-acceptor sites in these domains, we can observe the open/closed conformations upon reducing CYPOR in the presence and absence of cytochrome P450. Such study is important to better understand CYPOR mechanism of action in various endosomal membranes including hepatic CYPOR which is vital in plasma cholesterol homeostasis. By investigating the conformational cycles of CYPOR, we can synthesize drugs that would be more efficient in affecting the steroid hormonal levels in human cells.
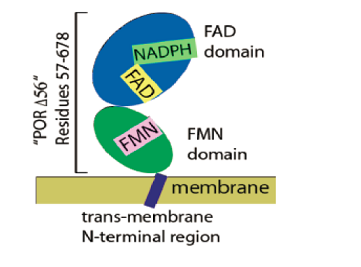
Figure 1: CYPOR has two parts : Cytosolic and membrane bound. There are two domains in the cytosolic part which are the FAD and FMN domains. CYPOR binds to membrane via its N-terminal region.
Daphne Mapolelo
University of Botswana, Botswana
Title: Synthesis and characterization of Ru(II)-bis-benzimidazole-based complexes, Ru(III)-polydentate pyridine-based complexes and Ru(II)-bis-benzimidazole polydentate pyridine-based complexes with broad spectrum antimicrobial activity and novel mode of action
Time : 15:30-15:55

Biography:
Daphne T Mapolelo has expertise in synthesis and spectroscopic characterization of ruthenium metal complexes. My current research interest lies in the synthesis, characterization and biological application of ruthenium polydentate pyridine and benzimidazole-based complexes. The characterization of these ruthenium complexes requires the use of a wide range of spectroscopic/magnetic techniques such as electron paramagnetic resonance (EPR), Nuclear Magnetic Resonance (NMR), MÖssbauer, resonance Raman, natural and magnetically induced circular dichroism (CD and MCD). In addition, in vitro and in vivo studies are employed to test the metal complexes for anticancer, anti HIV and antimicrobial activities.
Abstract:
We report herein the synthesis and characterization of Ru(II)-bis-benzimidazole-based complexes, Ru(III)- polydentate pyridine-based complexes and Ru(II)-bisbenzimidazole polydentate pyridine-based complexes respectively, being Ruthenium-aquo-1, 2-di(1Hbenzo[d]imidazole-2-yl) ethane trichloride [Ru(bbe)(H2O)Cl3], Ruthenium-tri-tert-butyl-2,2’,6,2’’- terpyridine-chloride-N,N,N’,N’-Tetrakis(pryidylmethyl)- 1,8 octanediamine perchlorate [Ru(terpy*)(1,8)Cl](ClO4)4 and Ruthenium-aquo-1, 2-di(1H-benzo[d]imidazole-2- yl)ethane chloride- N,N,N’,N’- Tetrakis- (pyridylmethyl)- 1,8-octanediamine perchlorate [Ru(bbe)(1,8)(H2O)Cl2](ClO4)4 respectively. Characterization of all the complexes was done by FTIR, 1 H-NMR, 13C-NMR spectroscopies, and Elemental Analysis. Cytotoxicity and antimicrobial activity studies against both gram negative and positive bacteria, as well as multi drug resistant bacterial isolates were tested for all the complexes. The CC50, EC50, MIC and possible mode of action for each metal complex were determined. Results showed that all metal complexes were not toxic to peripheral blood mononuclear cell (PBMCs), with calculated CC50 values ranging between 0.48 mg/mL to 1.0mg/mL. Antimicrobial properties of the complexes were determined by Kirby Bauer (KB) method and it was observed that both gram negative and positive bacteria, including multi drug resistant test microorganisms were susceptible. The calculated MICs ranged between 0.25- 2mg/L. The effective concentration (EC50) was between 0.18 mg/mL and 0.5mg/mL. Whereas the selective index (SI = CC50/EC50) was is 1.0 and 2.6 indicating low to high potential as microbial agent. The metal complexes were tested for possible mode of action and did not conform to known bactericidal activities suggesting a novel mechanism yet to be elucidated. !!!
Himanshu Ranjan Verma
Indian Institute of Technology (Banaras Hindu University), India
Title: A low temperature procedure for the delamination of waste printed circuit boards by dissolution of epoxy resin substrate
Time : 16:10-16:35

Biography:
Himanshu Ranjan Verma has his expertise in electronic/metallurgical waste recycling. He started his technical career by joining BTech in Mechanical Engg. After Graduation, he worked as Lecturer in a reputed engineering college and due to his inclination towards metallurgy he changed his domain of expertise to metallurgical Engg., during higher studies. He earned gold medal during his MTech (Metallurgical Engineering) from Indian Institute of Technology (BHU), Varanasi in 2013 and, immediately he has joined PhD at the same university. In-between, He worked in National Metallurgical Laboratory, Jamshedpur India, for 1 year as a Trainee pursuing research on rare-earth metal recycling. During his short research career of 3-4 years, he published 05 papers in high impact factor journals. He also participated in more than 12 conferences; secured awards like best poster, best presentation etc.
Abstract:
A new technique of waste printed circuit boards (WPCBs) recycling by dissolution of brominated epoxy resin (BER) in dimethyl formamide (DMF) has been investigated. Dissolution of BER is highly influenced by parameters like temperature, size, WPCB: DMF etc. and it results separation of layers of WPCBs. WPCBs of 1cm2, 2cm2 and 3cm2 were separated into components after 120 min, 180 min and 240 min respectively at 408 K and 300 g/l. DMF after usage was recycled and analyzed by gas chromatography, proton and carbon nuclear magnetic resonance spectroscopy. Residue obtained after recycling of DMF was analyzed by Fourier-transforms infra-red spectroscopy and scanning electron microscope-energy dispersive X-ray. Investigation of mechanism of dissolution revealed that H-bonding plays a very important role. This technique featuring separation of different components, regeneration of DMF, recovery of BER makes the process extremely cost effective, negligible effluent generating and thus, resulting cleaner processing of WPCBs.
Hadi Razavi-Khosroshahi
Nagoya Institute of Technology, Japan
Title: High-pressure titanium dioxide as visible-light driven photocatalyst with narrow bandgap
Time : 16:35-17:00

Biography:
Hadi Razavi-Khosroshahi has obtained his PhD in Materials Physics and Chemistry from Kyushu University, Fukuoka, Japan, in 2013. He is currently an Assistant Professor in Nagoya Institute of Technology, Japan. His main research interests are production of nanostructured metal oxides both from top-down and bottom-up approaches. He is recently working on narrowing the band gap of semiconductors by means of severe plastic deformation (SPD), and in particular by high-pressure torsion (HPT) method. His research results based on strain-induced phase transformations have revealed that high-pressure phases of various semiconductors are stabilized at ambient pressure are capable of being excellent environmental and energy materials like visible-light-active photocatalysts.
Abstract:
Titanium dioxide (TiO2) is a well-known semiconductor with superior photocatalytic properties. TiO2 has three polymorphs (anatase, rutile, and brookite) among which anatase exhibits the best photocatalytic properties because of its large electron effective mass, which leads to a low mobility of charge carriers. Despite its good photocatalytic features, application of pure anatase as a photocatalyst has been limited to the UV range of sunlight due to its wide bandgap (3.2 eV). Narrowing the bandgap of pure TiO2 using dopant-free approach has been in the spotlight in recent years. Theoretical studies suggest that high-pressure phases of TiO2 have narrower bandgaps which can coincide with the visible light. However, these phases are stable only under high pressures. High-pressure torsion (HPT) method is an effective technique for stabilizing high-pressure phases at ambient pressure. In this method, high pressure and large plastic strain are simultaneously applied to a sample between two rotating anvils. In this study, the HPT process was conducted at room temperature on pure (99.8%) anatase powder with an average particle size of ~150 nm. Photocatalytic hydrogen evolution was examined under UV light and under visible light. Moreover, X-ray diffraction, Raman spectroscopy, differential scanning calorimetry, photoluminescence spectroscopy, electron paramagnetic resonance analysis, electron energy loss spectroscopy, Fourier transform infrared spectroscopy, and UV-V diffuse reflectance spectroscopy are used for characterization of the material. Photocatalytic hydrogen generation by water splitting was examined as a measure of photocatalytic activity of TiO2.
Photocatalytic hydrogen generation as a function of time under UV and visible lights are shown in Figure1. Hydrogen generation rate under UV light is slower for the HPT-processed sample comparing with that for the anatase powder. However, the hydrogen generation rate is faster for the HPT-processed sample when illuminated by visible light. Hydrogen generation rate both under UV and visible lights significantly improves when the HPT-processed sample is annealed at 500°C.
Beena Jose
Vimala College, India
Title: Isolation and characterization of phytoconstituents and evaluation of anticancer potential of the medicinal plant Wrightia tinctoria (Roxb.) R. Br. from South India
Time : 17:00-17:25

Biography:
Beena Jose has completed her PhD in Chemistry from the University of Calicut in 2005. She has published 36 papers in various reputed national and international journals and authored one book. Her area of specialization is natural products chemistry. Currently she is working as an Assistant Professor in Chemistry at Vimala College, Thrissur, Kerala, India. She has presented papers in India and abroad. As a part of International Fellowship Program, she has been selected as an International Visiting Research Scholar at the Jesuit School of Theology (JST) of Santa Clara University, USA for the year 2014-15. She is the recipient of University Grants Commission’s (UGC) Major and Minor research projects. Her area of interests are phytochemical analysis and structural elucidation of the compounds isolated from plants.
Abstract:
Wrightia tinctoria R. Br. belongs to family Apocynaceae commonly called as “Jaundice curative tree” in South India. In Siddha system of medicine, it is used for psoriasis and other skin diseases. In the present study various secondary metabolites from the leaf and bark of Wrightia tinctoria (Pala Indigo) were isolated by column chromatography and characterized by spectral analysis (1H NMR, 13C NMR, IR, Mass Spectrum). The antibacterial and antifungal activities of the plant extracts against various pathogenic bacteria such as Bacillus cereus, Enterobacter faecalis, Salmonella paratyphi, Staphylococcus aureus, Escherichia coli, Proteus vulgaris, Klebsiella pneumoniae, Pseudomonas aeruginosa and Serratia marcescens and antifungal activity against two fungi namely Aspergillus niger and Penicillium chrysogenum were evaluated by ‘agar well diffusion method'. The anticancer potential of Wrightia tinctoria was studied both in vitro and in vivo. Anti-tumor properties of Wrightia tinctoria could be linked with the presence of antioxidants and cytotoxic activity. These outcomes indicate the possible potential use of Wrightia tinctoria as anti-tumor agent.
Heba M Adly
Umm Al-Qura University, Saudi Arabia
Title: Variations of some airborne trace elements concentrations as potential carcinogens
Time : 17:25-17:50

Biography:
Heba M Adly is an Assistant Professor of Environmental Health - Faculty of Medicine, Umm Al-Qura University [UQU] and Consultant of Environmental Health. Her technical expertise includes air modeling, environmental chemistry, gas chromatography, mass spectrometry and atomic absorption. She is a Member of American Environmental Laboratory Accreditation Institute [NELAC], Chemical Institute of Canada [CIC] and American Association for Clinical Chemistry [AACC]. She is a member of many academic and administrative committees at UQU. She teaches and participates in numerous curricula at the Faculty of Medicine and Medical Sciences. Currently, she is working as a Project Manager for reformed curricula project between UQU and UCL Medical School. She has two running research projects: (Assessment of cancer risk associated with long-term exposure of airborne some heavy metals in Makkah, KSA) and (assessment of environmental and occupational exposure to mercury among dental staff and its impact on antioxidant capacity).
Abstract:
Backgrounds: Particulate matter (PM10) may contain toxic trace elements that believed to be potent health risk factors due to their carcinogenicity. Makkah is the Muslim holy city in Saudi Arabia with a total population about 1.7 million, although it has limited industrial activities, it has unique characteristics every year, over 2.3 million of pilgrims stay in Makkah through hajj time which increase transportation pollution problems provoking undesignated amount of air elemental pollution.
Objective: This study aimed to determine the cancer risk for population exposed to four heavy metals (Cd, Cr, As, Be,) in ambient air.
Materials & Methods: The study was carried out in Arafat area, east of Makkah representing a highly-crowded area during Hajj time. Air samples were collected for 24 hours using mini volume sampler on a weekly base during summer and autumn 2014. Concentrations of PM10 trace elements (Cd, Cr, As, Be) were analyzed using ICP-MS. Since there were no carcinogenicity risk data of trace elements known in Makkah, measurement of cancer risk of each metal was calculated in accordance to US-EPA.
Results & conclusions: Atmospheric Cd, Cr, As concentrations were elevated in summer than autumn which are possibly due to high wind speed and temperature, an usual phenomenon in Saudi Arabia, leading to increase the atmospheric disturbance as a result of great amounts of dust resuspension from roadside and blowing sand particles. The cancer risk was found to be (1.08×10-4, 7.21×10-4, 4×10-6, 4.6×10-6) for Cd, Cr, As, Be respectively, transcending passable levels of inhalation risk for each element (10-6) as adopted by US EPA. This study may serve as reference to develop air quality management strategy of airborne trace elements related to cancer risk directly affect pilgrims.
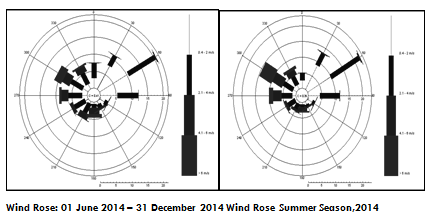
Wind roses for the Arafat area during the 2014 fall and summer seasons showing wind direction and the percentage of time that winds blew from a direction at certain speed ranges. Wind speeds shown in the plots are in m/s.
Ming Xia
Chinese Academy of Sciences, China
Title: Design, optimization and control of energy-saving dividing-wall column for separating azeotropes
Time : 17:50-18:15

Biography:
Ming Xia has received BS (2009) degree in Pharmaceutical Engineering from Zhengzhou University and PhD (2014) degree in Chemical Engineering from Tianjin University. After graduation in 2014/07, he has joined in the Institute of Coal Chemistry, Chinese Academy of Sciences, where he is now a research associate. His research interests involve resources, particularly focusing on novel reactor research and development (Equipment), steady-state design and dynamic control (Process), and industrial scale-up (plant/factory level), with the help of simulated and experimental methods. At present, he is focusing on the scale-up of multi-tubular fixed-bed reactor for Fischer-Tropsch synthesis (100,000 t/a liquid hydrocarbon), and methanol downstream technology, such as acetic acid to acetone and acetic acid to ethanol etc.
Abstract:
Dividing-wall column is an important way to process intensification. For the past decades, more and more publications were focused on the design and control of dividing-wall column (including petlyuk) since it usually offers considerable energy saving and investment reduction. However, the past research emphasized ideal/zoetropic separation. This work aims to extend the dividing-wall column to azeotrope separation for energy and investment benefits, with special focus on design, optimization and control. Firstly, this work reviewed the past research on the design and control of dividing-wall column. Then a sequential design, optimization and control procedure for azeotropic/extractive dividing-wall column was presented on the basis of total annual cost (TAC) and effective control criteria. At last, several cases were utilized to demonstrate the benefit potentials of azeotropic/extractive dividing-wall column.
(a) (b) 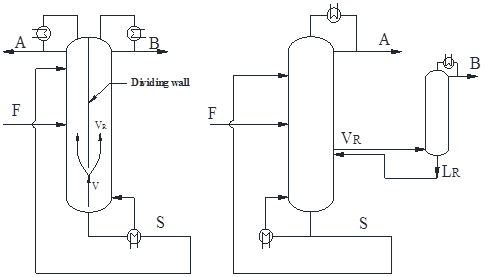
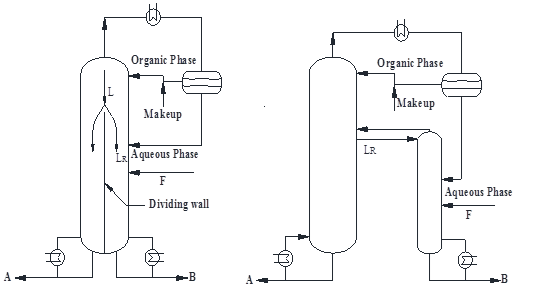 (c) (d)
(c) (d)
Figure.1: Extractive dividing-wall column: (a) Integration scheme, (b) Equivalent scheme, azeotropic dividing-wall column, (c) Integration scheme and (d) Equivalent scheme
Anthony Pembere
Chinese Academy of Sciences, China
Title: Jones oxidation of glycerol catalyzed by small gold clusters
Time : 18:15-18:40

Biography:
Abstract:
A joint theoretical and experimental study on the oxidation reactivity of glycerol catalyzed by chemically pure small Au clusters at the absence and presence of H2O2 was done. From high-resolution mass spectrometry, fruitful products of glyceraldehyde, glyceric acid, tartronic acid, mesoxalic acid and glycolic acid are observed pertaining to the successive Jones oxidation process associated with C-O and C–H bond activation. We then fully demonstrated the reaction pathways on a basis of complementary-active-sites mechanism, revealing the favorable dehydration of glycerol followed by oxidation to form glyceraldehyde and carboxylic acids at the presence of small Au clusters and H2O2. It is found that the Aun/H2O2 system undertakes a heterolytic mechanism by firstly transferring an O-atom from H2O2 to the Au cluster forming an active intermediate, on which the hydride abstraction and subsequent oxygen rebound become thermodynamically allowed, promoting the C-H insertion reaction and further oxidation of aldehyde to carboxylic acids.
Mahesh N Sanzgiri
St. Xaviers College, India
Title: Isolation, identification and field studies on tiger pheromones

Biography:
Mahesh N Sanzgiri has done his PhD in Organic Chemistry from Bombay University in 1984 under guidance of Prof. V V Nadkarny of St. Xavier’s College, Mumbai. His research is dedicated to study of few derivatives of anthrone and pheromones. His research work for application of derivatives of anthrone in anticancer and anti-aids activity is a new approach with synthesizing organic compounds using functional groups for trapping active cancer cells and removing aids activity. With wide experience in working with multinational German Chemical and Pharma Co. Merck in India for many years on responsible position, presently he works as a Freelance Scientist and Consultant for doing research and development, also consultant for cGMP, WHO, USFDA, ISO etc. with various well-known Indian and multinational Indian and foreign companies.
Abstract:
Isolation, identification and field study on tiger pheromones is concentrated in this work. Pheromones, although well-known in the insect world, not much work has been done in the case of mammals. In order to do the field studies, especially using this as a biochemical marker by tiger in territory marking and communication especially during mating season, the studies were carried out in forest. For the study, tiger urine was collected from different zoos in month of January, March, July and September to cover most of the seasons of the year. For separation of components of tiger urine for study purpose Merck HPTLC and preparative layer chromatographic techniques were used and so also electrophoratic run on Whatsmann’s paper. To confirm functional groups of components separated, IR Spectrum was used. Identification of structure of tiger pheromone molecular weight of tiger pheromone varies from tiger to tiger which indicates tiger pheromone is unique to each tiger. Due to this tiger marks its territory by spreading urine on trees which is identified by other tigers. Molecular weight varies anywhere between 1000-5000.
Parvesh Singh
1University of Kwa-Zulu Natal, South Africa
Title: Synthesis of functionalized-2-aryl-2, 3-dihydroquinoline-4(1H)-ones via Fries rearrangement of C-3 conjugated azetidin-2-ones

Biography:
Parvesh Singh received his PhD degree in Organic Chemistry from the Guru Nanak Dev University (India). Currently, he is working as a Senior Lecturer (Organic Chemistry) in the School of Chemistry and Physics at University of KwaZulu Natal (South Africa). His research interests involve the synthesis, biological evaluation and molecular modeling of heterocyclic scaffolds. He is primarily using hetero Diels-Alder methodology to synthesis heterocyclic rings of different sizes. Thus far, he has published 60 research articles in peer-reviewed journals of international repute including a book chapter and a book. Additionally, he has showcased his research work in several national and international conferences.
Abstract:
Quinoline-4-ones represent an important class of heterocyclic scaffolds that have attracted significant interest due to their various biological and pharmacological activities. This heterocyclic unit also constitutes an integral component in drugs used for the treatment of neurodegenerative diseases, sleep disorders and in antibiotics viz. norfloxacin and ciprofloxacin. The synthetic accessibility and possibility of fictionalization at varied positions in quinoline-4-ones exemplifies an elegant platform for the designing of combinatorial libraries of functionally enriched scaffolds with a range of pharmacological profles. They are also considered to be attractive precursors for the synthesis of medicinally imperative molecules such as non-steroidal androgen receptor antagonists, antimalarial drug chloroquine and martinellines with antibacterial activity. 2-Aryl-2, 3-dihydroquinolin-4(1H)-ones are present in many natural and non-natural compounds and are considered to be the aza-analogs of favanones. The b-lactam class of antibiotics is generally recognized to be a cornerstone of human health care due to the unparalleled clinical efficacy and safety of this type of antibacterial compound. In addition to their biological relevance as potential antibiotics, b-lactams have also acquired a prominent place in organic chemistry as synthons and provide highly efficient routes to a variety of non-protein amino acids, such as oligopeptides, peptidomimetics, nitrogen-heterocycles, as well as biologically active natural and unnatural products of medicinal interest such as indolizidine alkaloids, paclitaxel, docetaxel, taxoids, cyptophycins, lankacidins etc. A straight forward route toward the synthesis of quinoline-4-ones via the triflic acid assisted Fries rearrangement of N-aryl-blactams has been reported by Tepe and co-workers. The ring expansion observed in this case was solely attributed to the inherent ring strain in b-lactam ring because g-lactam failed to undergo rearrangement under reaction conditions. The above mentioned protocol has been recently extended by our group for the synthesis of benzo [b]-azocinon-6-ones via a tandem Michael addition–Fries rearrangement of sorbyl anilides as well as for the single-pot synthesis of 2-aryl-quinolin-4(3H)-ones through the Fries rearrangement of 3-dienyl-blactams. In continuation with our synthetic endeavors with the b-lactam ring and in view of the lack of convenient approaches for the synthesis of C-3 functionalized quinolin-4(1H)-ones, the present work describes the single-pot synthesis of C-3 functionalized quinolin-4(1H)-ones via the triflic acid promoted Fries rearrangement of C-3 vinyl/isopropenyl substituted b-lactams. In addition, DFT calculations and MD simulations were performed to investigate the stability profiles of synthetic compounds.
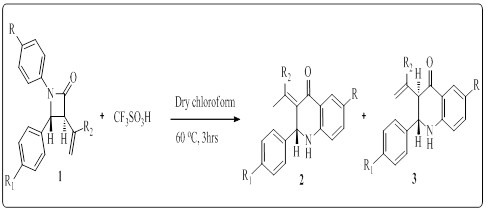
Sharad R Patil
Institute of Chemical Technology, India
Title: NIR-emitting quinone-fused coumarin dyes: Aqueous mediated, catalyst free synthesis and their optical properties

Biography:
Sharad R Patil has secured BSc (Hons) and MSc from North Maharashtra University, Jalgaon, Maharashtra, India. He was admitted to Institute of Chemical Technology, Mumbai for his PhD degree in the research group of Prof. N Sekar (CCol FSDC, FMASc). He completed his PhD degree in the year 2017. Immediately thereafter he joined UTU, Bardoli as an Assistant Professor in March 2017. He has 10 research publications in peer reviewed journals. His area of research includes study of organic reaction mechanisms, synthesis of heterocycles, biosensors, and green chemistry, dye sensitized solar cells (DSSC), computational chemistry, and color chemistry, NIR-absorbing/emitting molecules, to design and synthesize multi-specialty organic molecules and conjugated macromolecules or polymers for fluorescent sensors, research and development in chemistry for sustainability.
Abstract:
Water mediated organic synthesis is a fascinating approach in chemistry. We have attempted catalyst free synthesis of thermally stable near infra-red (NIR) emitting quinone fused coumarins with a benzothiazole/benzimidazole acceptor in aqueous medium. The synthesis was attempted using naturally occurring 2-hydroxy-1, 4-naphthoquinone. It is an efficient and environmentally friendly approach for the diversity oriented synthesis of 5, 6-quinone fused 2-pyrone containing compounds. It is a highly productive one pot synthetic method at room temperature that avails commercially accessible materials. In addition, this method has a very short reaction time and milder reaction conditions with an easy separation process. The composition of catalyst free and room temperature condition ensure a green approach towards the excellent practice of the synthetic method. These reactions offer functional NIR emitting fused coumarin compounds extended emission to 810 nm. Structural, spectroscopic and morphological characterization of the material confirms the purity, integrity and future potential materials for high technological applications. Eventually this method gives structurally interesting compounds having optical and pharmacological significance.
M A Salman
Kuwait Institute for Scientific Research, Kuwait
Title: A physical treatment method as a prevention method for barium sulfate scaling

Biography:
Abstract:
Barium sulfate (BaSO4) is a hard scaling usually precipitates on the surface of equipments in many industrial systems, as oil and gas production, desalination and cooling and boiler operation. It is a scale that is extremely resistance to both chemical and mechanical cleaning. So, BaSO4 is a problematic and expensive scaling. Although barium ions are present in most natural waters at very low concentration as low as 0.008 mg/l, it could result of scaling problems in the presence of high concentration of sulfate ion or when mixing with incompatible waters as in oil produced water. The scaling potential of BaSO4 will be investigated using seawater at the intake of seven desalination plants in Kuwait, brine water and Kuwait oil produced water. The best location in regards of barium sulfate scaling will be reported, then a physical treatment method (magnetic treatment method) will be used to control BaSO4 scaling using saturated solutions at different operating temperatures, flow velocities, feed pHs and different magnetic strength.
- Green Chemistry and Sustainable Technology
- Bioorganic and Biochemistry
- Organometallic Chemistry
- Inorganic and Bioinorganic Catalysis
- Polymer Chemistry
- Industrial Inorganic Chemistry
- Medicinal Chemistry, Drug Synthesis
- Physical Organic Chemistry
- Green Chemistry and Sustainable Technology
- Medicinal Chemistry, Drug Synthesis
- Advanced Synthesis and Catalysis
- Natural Products and Heterocyclic Chemistry
- Organic Chemical Engineering
- Modern Organic Chemistry and Applications
- Stereochemistry of Organic Compounds
- Natural Products and Heterocyclic Chemistry
- Advance Trends in Organic Chemistry
- Advance Trends in Organic Chemistry
- Inorganic Materials and Nanoparticles
